Transfer RNA Levels Are Tuned to Support Differentiation During Drosophila Neurogenesis
- PMID: 39766869
- PMCID: PMC11675937
- DOI: 10.3390/genes15121602
Transfer RNA Levels Are Tuned to Support Differentiation During Drosophila Neurogenesis
Abstract
Background/objectives: Neural differentiation requires a multifaceted program to alter gene expression along the proliferation to the differentiation axis. While critical changes occur at the level of transcription, post-transcriptional mechanisms allow fine-tuning of protein output. We investigated the role of tRNAs in regulating gene expression during neural differentiation in Drosophila larval brains.
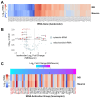
Methods: We quantified tRNA abundance in neural progenitor-biased and neuron-biased brains using the hydrotRNA-seq method. These tRNA data were combined with cell type-specific mRNA decay measurements and transcriptome profiles in order to model how tRNA abundance affects mRNA stability and translation efficiency.
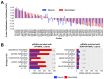
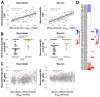
Results: We found that (1) tRNA abundance is largely constant between neural progenitors and neurons but significant variation exists for 10 nuclear tRNA genes and 8 corresponding anticodon groups, (2) tRNA abundance correlates with codon-mediated mRNA decay in neuroblasts and neurons, but does not completely explain the different stabilizing or destabilizing effects of certain codons, and (3) changes in tRNA levels support a shift in translation optimization from a program supporting proliferation to a program supporting differentiation.
Conclusions: These findings reveal coordination between tRNA expression and codon usage in transcripts that regulate neural development.
Keywords: codon usage; mRNA decay; mRNA translation; neurogenesis; tRNA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Update of
-
Transfer RNA levels are tuned to support differentiation during Drosophila neurogenesis.bioRxiv [Preprint]. 2024 Sep 6:2024.09.06.611608. doi: 10.1101/2024.09.06.611608. bioRxiv. 2024. Update in: Genes (Basel). 2024 Dec 15;15(12):1602. doi: 10.3390/genes15121602. PMID: 39282315 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

