Drought Stress Inhibits the Accumulation of Rotenoids and the Biosynthesis of Drought-Responsive Phytohormones in Mirabilis himalaica (Edgew.) Heim Calli
- PMID: 39766910
- PMCID: PMC11675678
- DOI: 10.3390/genes15121644
Drought Stress Inhibits the Accumulation of Rotenoids and the Biosynthesis of Drought-Responsive Phytohormones in Mirabilis himalaica (Edgew.) Heim Calli
Abstract
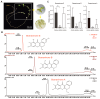
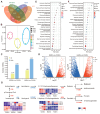
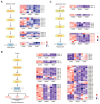
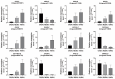
Background:Mirabilis himalaica, distributed in the high-altitude, arid, and semi-arid regions of Xizang, exhibits great tolerance to drought, which is rich in rotenoids and other secondary metabolites. It is still unknown, though, how drought stress influences rotenoid synthesis in M. himalaica. Methods: In this study, the calli of M. himalaica were subjected to 5% PEG6000 for 0, 20, and 40 h and divided into control group (CK), mild-drought-treated group (M), and high-drought-treated group (H), respectively. We then analyzed the relative content of three main rotenoids in M. himalaica using high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS). Results: Our findings demonstrated that the content of rotenoids was significantly reduced under drought stress. Transcriptome analysis subsequently revealed 14,525 differentially expressed genes (DEGs) between the different treatments. Furthermore, these DEGs exhibited enrichment in pathways associated with isoflavone biosynthesis and hormone signaling pathways. Key genes with decreased expression patterns during drought stress were also found to be involved in rotenoid accumulation and drought-responsive phytohormone signaling, including abscisic acid (ABA), auxin (IAA), and jasmonic acid (JA). Conclusions: These findings elucidate the molecular processes of drought resistance in M. himalaica and shed light on the relationship between rotenoid production and drought stress in M. himalaica.
Keywords: Mirabilis himalaica; abscisic acid; auxin; drought stress; jasmonate; rotenoids; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Integrated Analysis of Transcriptomic and Proteomics Data Reveals the Induction Effects of Rotenoid Biosynthesis of Mirabilis himalaica Caused by UV-B Radiation.Int J Mol Sci. 2018 Oct 25;19(11):3324. doi: 10.3390/ijms19113324. Int J Mol Sci. 2018. PMID: 30366418 Free PMC article.
-
Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in rotenoid biosynthesis in the medicinal plant Mirabilis himalaica.Mol Genet Genomics. 2018 Jun;293(3):635-647. doi: 10.1007/s00438-017-1409-y. Epub 2017 Dec 28. Mol Genet Genomics. 2018. PMID: 29285563 Free PMC article.
-
Identification of key genes and signaling pathways in coconut (Cocos nucifera L.) under drought stress via comparative transcriptome analysis.BMC Plant Biol. 2025 Apr 22;25(1):510. doi: 10.1186/s12870-025-06554-2. BMC Plant Biol. 2025. PMID: 40259217 Free PMC article.
-
JASMONATE ZIM-DOMAIN Family Proteins: Important Nodes in Jasmonic Acid-Abscisic Acid Crosstalk for Regulating Plant Response to Drought.Curr Protein Pept Sci. 2021 Dec 29;22(11):759-766. doi: 10.2174/1389203722666211018114443. Curr Protein Pept Sci. 2021. PMID: 34666642 Review.
-
Phytohormones enhanced drought tolerance in plants: a coping strategy.Environ Sci Pollut Res Int. 2018 Nov;25(33):33103-33118. doi: 10.1007/s11356-018-3364-5. Epub 2018 Oct 3. Environ Sci Pollut Res Int. 2018. PMID: 30284160 Review.
References
-
- Borrelli F., Milic N., Ascione V., Capasso R., Izzo A.A., Capasso F., Petrucci F., Valente R., Fattorusso E., Taglialatela-Scafati O. Isolation of new rotenoids from Boerhaavia Diffusa and evaluation of their effect on intestinal motility. Planta Med. 2005;71:928–932. doi: 10.1055/s-2005-871282. - DOI - PubMed
-
- Gu L., Zhang Z., Quan H., Li M., Zhao F., Xu Y., Liu J., Sai M., Zheng W., Lan X. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in rotenoid biosynthesis in the medicinal plant Mirabilis himalaica. Mol. Genet. Genom. 2018;293:635–647. doi: 10.1007/s00438-017-1409-y. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

