Mitigating Oxidative Stress and Promoting Cellular Longevity with Mushroom Extracts
- PMID: 39766971
- PMCID: PMC11727512
- DOI: 10.3390/foods13244028
Mitigating Oxidative Stress and Promoting Cellular Longevity with Mushroom Extracts
Abstract
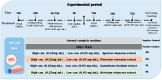
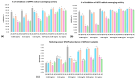
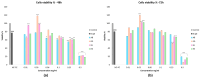
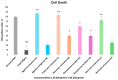
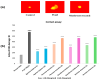
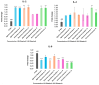
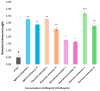
Oxidative stress can disrupt the body's ability to fight harmful free radicals, leading to premature aging and various health complications. This study investigated the antioxidant and anti-aging properties of four medicinal and edible mushrooms: Ganoderma lucidum, Hericium erinaceus, Pleurotus ostreatus, and Agaricus bisporus. The antioxidant activity of mushroom extracts was evaluated using (DPPH-ABTS-Reducing power). The anti-aging effects were assessed using Human Skin Fibroblasts (HSF) cells subjected to D-galactose-induced aging (30 g/L/72 h) and treated with mushroom extracts (0.03-0.25 mg/mL/72 h). The results demonstrated that all mushrooms have significant antioxidant and anti-aging properties, with low concentrations of extracts (0.03 mg/mL) effectively promoting cell proliferation at an 87% rate in the Agaricus bisporus extract, enhancing cell cycle progression by reducing the arrested cells in the G0/G1 phase to 75%, and promoting DNA synthesis in S phase by more than 16.36% in the Hericium erinaceus extract. Additionally, the extracts reduced DNA damage and Reactive Oxygen Species (ROS) levels, protecting cells from oxidative stress and potentially contributing to anti-aging effects. The mushrooms also exhibited immunomodulatory and anti-inflammatory effects by upregulating the IL-2, IL-4, and downregulating IL-6 expression, indicating their potential to promote general health. These findings suggest the potential of mushroom extracts as natural agents for reducing the negative effects of aging while promoting cellular health. Further research is required to explore the specific bioactive compounds responsible for these beneficial effects and to evaluate their efficacy in vivo.
Keywords: D-gal; DNA damage; IL-genes; ROS; anti-aging; antioxidants; cell cycle; mushroom; telomere length.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures






References
-
- Mallikarjuna S.E., Ranjini A., Haware D.J., Vijayalakshmi M.R., Shashirekha M.N., Rajarathnam S. Mineral Composition of Four Edible Mushrooms. J. Chem. 2013;2013:805284. doi: 10.1155/2013/805284. - DOI
-
- Shibu M.A., Agrawal D.C., Huang C.-Y. Springer; Singapore: 2017. Mushrooms: A Pandora Box of Cardioprotective Phytochemicals. Medicinal Plants and Fungi: Recent Advances in Research and Development.
Grants and funding
- 22001XA/the Special Project for the Protection and Utilization of Agricultural Resources of the Department of Agriculture and Rural Affairs of Fujian Province, "Research and Application of Key Technologies for Innovation and Industrialized Utilization of Juncao G
- 2019GBJ003216/China Scholarship Council
LinkOut - more resources
Full Text Sources

