Development of a Dual-Readout Multicolor Immunoassay for the Rapid Analysis of Isocarbophos in Vegetable and Fruit Samples
- PMID: 39766999
- PMCID: PMC11675379
- DOI: 10.3390/foods13244057
Development of a Dual-Readout Multicolor Immunoassay for the Rapid Analysis of Isocarbophos in Vegetable and Fruit Samples
Abstract
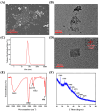
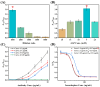
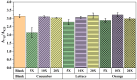
Multicolor immunoassay is a powerful tool for rapid analysis without the use of bulky instruments owing to various color conversions, which is suitable for on-site visual analysis for pesticides. Herein, this study developed a multicolor immunoassay for the rapid detection of isocarbophos. After competitive immunoassay, the secondary antibody (GAM-ALP) catalyzed ascorbyl-2-phosphate (AAP) into ascorbic acid (AA). The AA can reduce K3[Fe(CN)6] into K4[Fe(CN)6]. The latter can react with Fe3+ to form Prussian blue; otherwise, the orange AAP-Fe3+ complex was generated. Therefore, the multicolor immunoassay achieved a color conversion of orange-green-blue in response to isocarbophos, allowing for rapid semiquantitative analysis by the naked eye. After parameter optimization, the multicolor immunoassay was developed depending on the ratiometric absorbance between the Prussian blue and AAP-Fe3+ complex. Moreover, a smartphone was used to measure the RGB value of the color conversion for the development of portable visual, quantitative analysis. Both the absorbance-based and RGB-based multicolor immunoassays showed good accuracy and practicability in the recovery test. This study provided a common approach for the development of dual-readout multicolor immunoassay, which can be used for on-site rapid screening by quantitative or visual semiquantitative analysis.
Keywords: RGB analysis; alkaline phosphase; isocarbophos; multicolor immunoassay; pesticide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Simple dual-readout immunosensor based on phosphate-triggered and potassium permanganate for visual detection of fenitrothion.Biosens Bioelectron. 2024 Feb 15;246:115872. doi: 10.1016/j.bios.2023.115872. Epub 2023 Nov 25. Biosens Bioelectron. 2024. PMID: 38039731
-
A high-resolution colorimetric immunoassay platform realized by coupling enzymatic multicolor generation with smartphone readout.Analyst. 2018 Jun 11;143(12):2901-2907. doi: 10.1039/c8an00382c. Analyst. 2018. PMID: 29808208
-
A concentration-dependent multicolor conversion strategy for ultrasensitive colorimetric immunoassay with the naked eye.Anal Chim Acta. 2017 Apr 22;963:129-135. doi: 10.1016/j.aca.2017.01.034. Epub 2017 Jan 31. Anal Chim Acta. 2017. PMID: 28335966
-
Alkaline phosphatase-regulated in situ formation of chromogenic probes for multicolor visual sensing of biomarkers.Talanta. 2021 Jun 1;228:122222. doi: 10.1016/j.talanta.2021.122222. Epub 2021 Feb 19. Talanta. 2021. PMID: 33773728
-
Noble Metal Nanoparticle-Based Multicolor Immunoassays: An Approach toward Visual Quantification of the Analytes with the Naked Eye.ACS Sens. 2019 Apr 26;4(4):782-791. doi: 10.1021/acssensors.9b00438. Epub 2019 Apr 4. ACS Sens. 2019. PMID: 30896159 Review.
References
-
- Zhao F.N., Wang L., Li M.Y., Wang M., Liu G.Y., Ping J.F. Nanozyme-based biosensor for organophosphorus pesticide monitoring: Functional design, biosensing strategy, and detection application. TrAC Trends Anal. Chem. 2023;165:117152. doi: 10.1016/j.trac.2023.117152. - DOI
Grants and funding
- KF202402/Key Laboratory of Tropical Fruit and Vegetable Cold-chain of Hainan Province
- 32402222/National Natural Science Foundation of China
- 2020ZDZX2045/the Science Project of Department of Education of Guangdong Province
- Z823280-10/Fujian Provincial Key Laboratory of Food Microbiology and Enzyme Engineering
- 2023040308004/the Science and Technology Innovation Project of Zhaoqing City
LinkOut - more resources
Full Text Sources
Miscellaneous

