Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid
- PMID: 39767576
- PMCID: PMC11726740
- DOI: 10.3390/biomedicines12122669
Age-Related Choroidal Involution Is Associated with the Senescence of Endothelial Progenitor Cells in the Choroid
Abstract
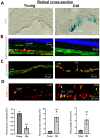
Background: Choroidal involution is a common feature of age-related ischemic retinopathies such as age-related macular degeneration (AMD). It is now well recognized that endothelial progenitor cells (EPCs) are essential to endothelial repair processes and in maintaining vascular integrity. However, the contribution of EPCs and the role of senescence in age-related choroidal vascular degeneration remain to be investigated. In this study, we compared the senescent phenotype of EPCs in the choroid and performed whole-genome profiling of EPCs derived from young versus old rats. Methods and Results: We isolated and compared the retinas of young (6-weeks-old) and old (16-18-month-old) rats. The thickness of the choroid and outer nuclear layer (ONL), along with local quantification of CD34+ EPCs, was performed. Compared to young rats, older rats displayed a significant reduction in choroidal and ONL thickness associated with markedly fewer choroid-localized EPCs; this was attested by lower expression of several EPC markers (CXCR4, CD34, CD117, CD133, and KLF-2). Choroid and choroid-localized EPCs displayed abundant senescence as revealed by increased β-gal and P53 expression and decreased Lamin-B1 (immunostaining and RT-qPCR). Concordantly, choroidal cells and EPCs isolated from older rats were unable to form vascular networks ex vivo. To better understand the potential mechanisms associated with the dysfunctional EPCs linked to age-related choroidal involution, we performed whole-genome profiling (mRNA and miRNA) of EPCs derived from old and young rats using next-generation sequencing (NGS); 802 genes were significantly modulated in old vs. young EPCs, corresponding to ~2% of total genes expressed. Using a bioinformatic algorithm, the KEGG pathways suggested that these genes participate in the modulation of several key signaling processes including inflammation, G protein-coupled receptors, and hematopoietic cell lineages. Moreover, we identified 13 miRNAs involved in the regulation of immune system processes, cell cycle arrest and senescence, which are significantly modulated in EPCs from old rats compared to young ones. Conclusions: Our results suggest that age-related choroidal involution is associated with fewer EPCs, albeit displaying a senescence-like phenotype. One would be tempted to propose that biological modification of native EPCs (such as with senolytic agents) could potentially provide a new strategy to preserve the vascular integrity of the aged choroid, and evade progression to degenerative maculopathies.
Keywords: aging; choroidal involution; endothelial progenitor cell (EPC); senescence; vascular network.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




References
-
- Harris A., Bingaman D., Ciulla T.A., Martin B. Chapter 5—Retinal and Choroidal Blood Flow in Health and Disease. In: Ryan S.J., Hinton D.R., Schachat A.P., Wilkinson C.P., editors. Retina. 4th ed. Mosby; Edinburgh, UK: 2006. pp. 83–102. - DOI
-
- Zhou T.E., Rivera J.C., Bhosle V.K., Lahaie I., Shao Z., Tahiri H., Zhu T., Polosa A., Dorfman A., Beaudry-Richard A., et al. Choroidal Involution Is Associated with a Progressive Degeneration of the Outer Retinal Function in a Model of Retinopathy of Prematurity: Early Role for IL-1β. Am. J. Pathol. 2016;186:3100–3116. doi: 10.1016/j.ajpath.2016.08.004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

