Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut-Brain Axis: Implications for Alzheimer's and Parkinson's Disease Pathogenesis
- PMID: 39767577
- PMCID: PMC11727295
- DOI: 10.3390/biomedicines12122670
Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut-Brain Axis: Implications for Alzheimer's and Parkinson's Disease Pathogenesis
Abstract
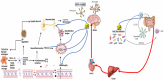
Microbiota-derived hydrogen sulfide (H2S) plays a crucial role in modulating the gut-brain axis, with significant implications for neurodegenerative diseases such as Alzheimer's and Parkinson's. H2S is produced by sulfate-reducing bacteria in the gut and acts as a critical signaling molecule influencing brain health via various pathways, including regulating inflammation, oxidative stress, and immune responses. H2S maintains gut barrier integrity at physiological levels and prevents systemic inflammation, which could impact neuroinflammation. However, as H2S has a dual role or a Janus face, excessive H2S production, often resulting from gut dysbiosis, can compromise the intestinal barrier and exacerbate neurodegenerative processes by promoting neuroinflammation and glial cell dysfunction. This imbalance is linked to the early pathogenesis of Alzheimer's and Parkinson's diseases, where the overproduction of H2S exacerbates beta-amyloid deposition, tau hyperphosphorylation, and alpha-synuclein aggregation, driving neuroinflammatory responses and neuronal damage. Targeting gut microbiota to restore H2S homeostasis through dietary interventions, probiotics, prebiotics, and fecal microbiota transplantation presents a promising therapeutic approach. By rebalancing the microbiota-derived H2S, these strategies may mitigate neurodegeneration and offer novel treatments for Alzheimer's and Parkinson's diseases, underscoring the critical role of the gut-brain axis in maintaining central nervous system health.
Keywords: Alzheimer’s disease; H2S; Parkinson’s disease; dysbiosis; glial cell dysfunction; gut–brain axis; microbiota; neurodegeneration; neuroinflammation; prebiotics; probiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

