Polymeric Nanoparticles Potentiate the Anticancer Activity of Novel PI3Kα Inhibitors Against Triple-Negative Breast Cancer Cells
- PMID: 39767583
- PMCID: PMC11727162
- DOI: 10.3390/biomedicines12122676
Polymeric Nanoparticles Potentiate the Anticancer Activity of Novel PI3Kα Inhibitors Against Triple-Negative Breast Cancer Cells
Abstract
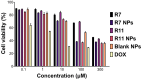
Background: Dysregulation in phosphoinositide-3-kinase alpha (PI3Kα) signaling is implicated in the development of various cancers, including triple-negative breast cancer (TNBC). We have previously synthesized a series of N-phenyl-6-chloro-4-hydroxy-2-quinolone-3-carboxamides as targeted inhibitors against PI3Kα. Herein, two drug candidates, R7 and R11, were selected to be further investigated as a nanoparticle (NP) formulation against TNBC. Methods: R7 and R11 were entrapped in D-α-tocopheryl poly(ethylene glycol) 1000 succinate (TPGS) polymeric NPs by nanoprecipitation. Following their physicochemical characterization, the anticancer activity of the compounds and their NP formulations was evaluated in the TNBC cell line MDA-MB-231 by conducting viability, uptake, and apoptosis assays, as well as penetration assays in a multicellular tumor spheroid model. Results: The NPs exhibited a particle size of 100-200 nm, excellent drug loading efficiencies, and sustained release under physiologic conditions. Viability assays revealed superior potency for the NP formulations, with IC50 values of 20 µM and 30 µM for R7- and R11-loaded NPs, respectively, compared to the free compounds, which exhibited IC50 values of 280 µM and 290 µM for R7 and R11, respectively. These results were attributed to the inherent antiproliferative activity of TPGS, as evidenced by the cytotoxicity of the drug-free NPs, as well as the enhanced cellular uptake enabled by the NP vehicle, as demonstrated by fluorescence microscopy imaging and flow cytometry measurements. Further investigations showed that the NPs promoted apoptosis via a mitochondrial-dependent pathway that involved the activation of proapoptotic caspases. Moreover, the NP formulations enhanced the penetration ability of the free compounds in multicellular tumor spheroids, causing a time- and concentration-dependent disruption of the spheroids. Conclusions: Our findings highlight the important role nanotechnology can play in improving the biopharmaceutical properties of new drug candidates and facilitating their in vivo translation.
Keywords: PI3Kα; TPGS; polymeric nanoparticles; protein kinases; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- WHO Cancer. [(accessed on 16 November 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
-
- WHO Breast Cancer. [(accessed on 15 November 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

