Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results
- PMID: 39767669
- PMCID: PMC11672947
- DOI: 10.3390/biomedicines12122762
Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results
Abstract
Background: Current treatments for hypertrophic obstructive cardiomyopathy (HOCM), including medication, surgery, and alcohol septal ablation (ASA), have limitations in terms of efficacy and safety. To address these challenges, we developed the trans-coronary intra-septal radiofrequency ablation (TIRA) device.
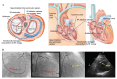
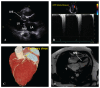
Methods: This first-in-human trial was conducted to assess the efficacy and safety of the TIRA device. Moreover, evaluations were conducted before the procedure and at 3, 6, and 12 months post-procedure using computed tomography, magnetic resonance imaging, echocardiography, and the 6 min walk distance (6MWD) test.
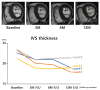
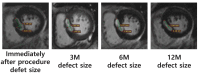
Results: Four patients were enrolled, and follow-up imaging at 3, 6, and 12 months showed a reduction in the interventricular septal (IVS) thickness (baseline mean: 22.6 mm; 12-month mean: 18.9 mm) and a decrease in the LVOT pressure gradient at 12 months (resting baseline mean: 84.64 mmHg; resting 12-month mean: 43.56 mmHg; Valsalva baseline mean: 129.96 mmHg; Valsalva 12-month mean: 108.16 mmHg). However, reductions in the IVS thickness on echocardiography and improvements in 6MWD were observed in only two patients.
Conclusions: No significant adverse events, such as arrhythmias or vascular injuries, were reported. These findings suggest that the TIRA device may be a safe and effective option for treating HOCM. However, further studies are required to confirm these results.
Keywords: TIRA-HOCM device; coronary venous system access; first in-human clinical trial; hypertrophic obstructive cardiomyopathy (HOCM); left ventricular outflow tract (LVOT) obstruction.
Conflict of interest statement
The corresponding author (Min-Ku Chon) has intellectual property of the spacer device, stock of TAU MEDICAL, is currently working as the clinical director of TAU MEDICAL Inc. The other authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Medical

