Fucoidan Attenuates Cardiac Remodeling by Inhibiting Galectin-3 Secretion, Fibrosis, and Inflammation in a Mouse Model of Pressure Overload
- PMID: 39767753
- PMCID: PMC11673818
- DOI: 10.3390/biomedicines12122847
Fucoidan Attenuates Cardiac Remodeling by Inhibiting Galectin-3 Secretion, Fibrosis, and Inflammation in a Mouse Model of Pressure Overload
Abstract
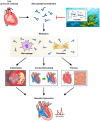
Background/objectives: Fucoidan, a sulfated polysaccharide derived from marine algae, is known for its antioxidant and immunomodulatory properties. Galectin-3 (Gal-3), a protein associated with cardiovascular fibrosis, has been identified as a potential therapeutic target in cardiac remodeling. This study aimed to evaluate whether fucoidan could inhibit Gal-3 activity and mitigate cardiac remodeling in a mouse model of pressure overload-induced cardiac hypertrophy.
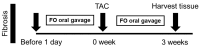
Methods: To test this hypothesis, we used transverse aortic constriction (TAC) surgery to induce pressure overload in normotensive mice, replicating the pathological features of cardiac hypertrophy. Mice were treated with fucoidan at a dose of 1.5 or 7.5 mg/kg/day. In vivo assessments of cardiac function, fibrosis, inflammation, and Gal-3 expression were performed.
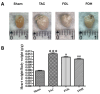
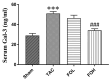
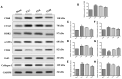
Results: Pressure overload led to significant upregulation of serum Gal-3 levels, increased cardiac collagen deposition, and elevated markers of fibrosis and inflammation. In mice treated with fucoidan, these effects were significantly attenuated. Fucoidan treatment prevented the upregulation of Gal-3, reduced collagen deposition, and decreased inflammatory cell infiltration, suggesting an inhibition of both fibrosis and inflammation.
Conclusions: Fucoidan effectively mitigated the adverse effects of pressure overload in this mouse model, including reduced Gal-3 expression, fibrosis, and inflammation. These findings suggest that fucoidan holds promise as a therapeutic agent for preventing or delaying cardiac remodeling and associated complications, such as fibrosis and inflammation, in pressure overload-induced cardiac hypertrophy. Further research is needed to explore the underlying mechanisms and clinical applicability of fucoidan in cardiac disease.
Keywords: fibrosis; fucoidan; galectin-3; pressure overload.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Martínez-Martínez E., Calvier L., Fernández-Celis A., Rousseau E., Jurado-López R., Rossoni L.V., Jaisser F., Zannad F., Rossignol P., Cachofeiro V., et al. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension. 2015;66:767–775. doi: 10.1161/HYPERTENSIONAHA.115.05876. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

