Navigating Hemorrhagic Shock: Biomarkers, Therapies, and Challenges in Clinical Care
- PMID: 39767770
- PMCID: PMC11673713
- DOI: 10.3390/biomedicines12122864
Navigating Hemorrhagic Shock: Biomarkers, Therapies, and Challenges in Clinical Care
Abstract
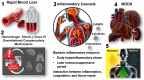
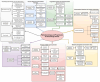
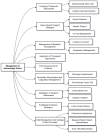
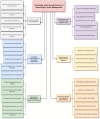
Hemorrhagic shock remains a leading cause of preventable death worldwide, with mortality patterns varying significantly based on injury mechanisms and severity. This comprehensive review examines the complex pathophysiology of hemorrhagic shock, focusing on the temporal evolution of inflammatory responses, biomarker utility, and evidence-based therapeutic interventions. The inflammatory cascade progresses through distinct phases, beginning with tissue injury and endothelial activation, followed by a systemic inflammatory response that can transition to devastating immunosuppression. Recent advances have revealed pattern-specific responses between penetrating and blunt trauma, necessitating tailored therapeutic approaches. While damage control resuscitation principles and balanced blood product administration have improved outcomes, many molecular targeted therapies remain investigational. Current evidence supports early hemorrhage control, appropriate blood product ratios, and time-sensitive interventions like tranexamic acid administration. However, challenges persist in biomarker validation, therapeutic timing, and implementation of personalized treatment strategies. Future directions include developing precision medicine approaches, real-time monitoring systems, and novel therapeutic modalities while addressing practical implementation barriers across different healthcare settings. Success in hemorrhagic shock management increasingly depends on integrating multiple interventions across different time points while maintaining focus on patient-centered outcomes.
Keywords: coagulopathy; damage control; hemorrhagic shock; inflammation; trauma resuscitation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Accreditation through the eyes of nurse managers: an infinite staircase or a phenomenon that evaporates like water.J Health Organ Manag. 2025 Jun 30. doi: 10.1108/JHOM-01-2025-0029. Online ahead of print. J Health Organ Manag. 2025. PMID: 40574247
-
Hail Lifestyle Medicine consensus position statement as a medical specialty: Middle Eastern perspective.Front Public Health. 2025 Jun 20;13:1455871. doi: 10.3389/fpubh.2025.1455871. eCollection 2025. Front Public Health. 2025. PMID: 40620567 Free PMC article.
-
The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review.Health Technol Assess. 2012;16(19):1-316. doi: 10.3310/hta16190. Health Technol Assess. 2012. PMID: 22480797 Free PMC article.
Cited by
-
Applications of Hydrogels in Emergency Therapy.Gels. 2025 Mar 23;11(4):234. doi: 10.3390/gels11040234. Gels. 2025. PMID: 40277670 Free PMC article. Review.
References
Publication types
Grants and funding
- 1R01GM153949/National Institutes of Health, (NIH); National Institute of General Medical Sciences (NIGMS)
- K08GM134185/National Institutes of Health, (NIH); National Institute of General Medical Sciences (NIGMS)
- R01 GM153949/GM/NIGMS NIH HHS/United States
- K08 GM134185/GM/NIGMS NIH HHS/United States
- R35 GM131831/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

