Flow-Dependent Modulation of Endothelial Ca2+ Dynamics by Small Conductance Ca2+-Activated K+ Channels in Mouse Carotid Arteries
- PMID: 39767806
- PMCID: PMC11727411
- DOI: 10.3390/biomedicines12122900
Flow-Dependent Modulation of Endothelial Ca2+ Dynamics by Small Conductance Ca2+-Activated K+ Channels in Mouse Carotid Arteries
Abstract
Background: Small conductance Ca2+ activated K+ channels (KCa2.3) are important regulators of vascular function. They provide Ca2+-dependent hyperpolarization of the endothelial membrane potential, promoting agonist-induced vasodilation. Another important mechanism of influence may occur through positive feedback regulation of endothelial Ca2+ signals, likely via amplification of influx through membrane cation channels. KCa2.3 channels have recently been implicated in flow-mediated dilation of the arterial vasculature and may contribute to the crucial homeostatic role of shear stress in preventing vascular wall remodeling and progressive vascular disease (i.e., atherosclerosis). The impact of KCa2.3 channels on endothelial Ca2+ signaling under physiologically relevant shear stress conditions remains unknown.
Methods: In the current study, we employ mice expressing an endothelium-specific Ca2+ fluorophore (cdh5-GCaMP8) to characterize the KCa2.3 channel influence on the dynamic Ca2+ signaling profile along the arterial endothelium in the presence and absence of shear-stress.
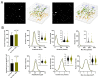
Results: Our data indicate KCa2.3 channels have a minimal influence on basal Ca2+ signaling in the carotid artery endothelium in the absence of flow, but they contribute substantially to amplification of Ca2+ dynamics in the presence of flow and their influence can be augmented through exogenous positive modulation.
Conclusions: The findings suggest a pivotal role for KCa2.3 channels in adjusting the profile of homeostatic dynamic Ca2+ signals along the arterial intima under flow.
Keywords: Ca2+ activated K+ channels; calcium; endothelium; shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ledoux J., Taylor M.S., Bonev A.D., Hannah R.M., Solodushko V., Shui B., Tallini Y., Kotlikoff M.I., Nelson M.T. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

