Pervasiveness of Microprotein Function Amongst Drosophila Small Open Reading Frames (SMORFS)
- PMID: 39768181
- PMCID: PMC11674832
- DOI: 10.3390/cells13242090
Pervasiveness of Microprotein Function Amongst Drosophila Small Open Reading Frames (SMORFS)
Abstract
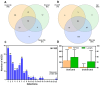
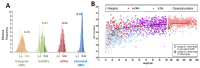
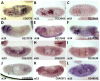
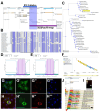
Small Open Reading Frames (smORFs) of less than 100 codons remain mostly uncharacterised. About a thousand smORFs per genome encode peptides and microproteins about 70-80 aa long, often containing recognisable protein structures and markers of translation, and these are referred to as short Coding Sequences (sCDSs). The characterisation of individual sCDSs has provided examples of smORFs' function and conservation, but we cannot infer the functionality of all other metazoan smORFs from these. sCDS function has been characterised at a genome-wide scale in yeast and bacteria, showing that hundreds can produce a phenotype, but attempts in metazoans have been less successful. Either most sCDSs are not functional, or classic experimental techniques do not work with smORFs due to their shortness. Here, we combine extensive proteomics with bioinformatics and genetics in order to detect and corroborate sCDS function in Drosophila. Our studies nearly double the number of sCDSs with detected peptides and microproteins and an experimentally corroborated function. Finally, we observe a correlation between proven sCDS protein function and bioinformatic markers such as conservation and GC content. Our results support that sCDSs peptides and microproteins act as membrane-related regulators of canonical proteins, regulators whose functions are best understood at the cellular level, and whose mutants produce little, if any, overt morphological phenotypes.
Keywords: Drosophila melanogaster; autophagy regulation; embryogenesis; functional genomics; microproteins; proteomics; ribosome profiling; sCDS (short coding sequences); smORFs (small open reading frames).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

