Three-Dimensional Ultrasound for Physical and Virtual Fetal Heart Models: Current Status and Future Perspectives
- PMID: 39768529
- PMCID: PMC11679263
- DOI: 10.3390/jcm13247605
Three-Dimensional Ultrasound for Physical and Virtual Fetal Heart Models: Current Status and Future Perspectives
Abstract
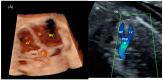
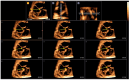
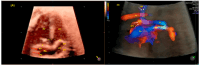
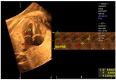
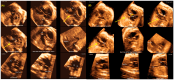
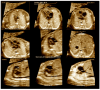
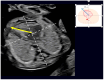
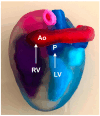
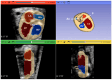
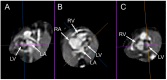
Congenital heart defects (CHDs) are the most common congenital defect, occurring in approximately 1 in 100 live births and being a leading cause of perinatal morbidity and mortality. Of note, approximately 25% of these defects are classified as critical, requiring immediate postnatal care by pediatric cardiology and neonatal cardiac surgery teams. Consequently, early and accurate diagnosis of CHD is key to proper prenatal and postnatal monitoring in a tertiary care setting. In this scenario, fetal echocardiography is considered the gold standard imaging ultrasound method for the diagnosis of CHD. However, the availability of this examination in clinical practice remains limited due to the need for a qualified specialist in pediatric cardiology. Moreover, in light of the relatively low prevalence of CHD among at-risk populations (approximately 10%), ultrasound cardiac screening for potential cardiac anomalies during routine second-trimester obstetric ultrasound scans represents a pivotal aspect of diagnosing CHD. In order to maximize the accuracy of CHD diagnoses, the views of the ventricular outflow tract and the superior mediastinum were added to the four-chamber view of the fetal heart for routine ultrasound screening according to international guidelines. In this context, four-dimensional spatio-temporal image correlation software (STIC) was developed in the early 2000s. Some of the advantages of STIC in fetal cardiac evaluation include the enrichment of anatomical details of fetal cardiac images in the absence of the pregnant woman and the ability to send volumes for analysis by an expert in fetal cardiology by an internet link. Sequentially, new technologies have been developed, such as fetal intelligent navigation echocardiography (FINE), also known as "5D heart", in which the nine fetal cardiac views recommended during a fetal echocardiogram are automatically generated from the acquisition of a cardiac volume. Furthermore, artificial intelligence (AI) has recently emerged as a promising technological innovation, offering the potential to warn of possible cardiac anomalies and thus increase the ability of non-cardiology specialists to diagnose CHD. In the early 2010s, the advent of 3D reconstruction software combined with high-definition printers enabled the virtual and 3D physical reconstruction of the fetal heart. The 3D physical models may improve parental counseling of fetal CHD, maternal-fetal interaction in cases of blind pregnant women, and interactive discussions among multidisciplinary health teams. In addition, the 3D physical and virtual models can be an useful tool for teaching cardiovascular anatomy and to optimize surgical planning, enabling simulation rooms for surgical procedures. Therefore, in this review, the authors discuss advanced image technologies that may optimize prenatal diagnoses of CHDs.
Keywords: artificial intelligence; congenital heart disease; fetal heart; fetal intelligent navigation echocardiography; spatiotemporal image correlation; three-dimensional ultrasound; ultrasonography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Fetal Intelligent Navigation Echocardiography (FINE): a novel method for rapid, simple, and automatic examination of the fetal heart.Ultrasound Obstet Gynecol. 2013 Sep;42(3):268-84. doi: 10.1002/uog.12563. Ultrasound Obstet Gynecol. 2013. PMID: 24000158 Free PMC article.
-
Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart.Ultrasound Obstet Gynecol. 2017 Oct;50(4):476-491. doi: 10.1002/uog.17522. Epub 2017 Aug 14. Ultrasound Obstet Gynecol. 2017. PMID: 28809063 Free PMC article.
-
First-trimester fetal cardiac examination using spatiotemporal image correlation, tomographic ultrasound and color Doppler imaging for the diagnosis of complex congenital heart disease in high-risk patients.Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7. doi: 10.1002/uog.13341. Epub 2014 Oct 15. Ultrasound Obstet Gynecol. 2014. PMID: 24585667
-
Evolution of Fetal Cardiac Imaging over the Last 20 Years.Diagnostics (Basel). 2023 Nov 22;13(23):3509. doi: 10.3390/diagnostics13233509. Diagnostics (Basel). 2023. PMID: 38066750 Free PMC article. Review.
-
A "holistic" sonographic view on congenital heart disease: How automatic reconstruction using fetal intelligent navigation echocardiography eases unveiling of abnormal cardiac anatomy part II-Left heart anomalies.Echocardiography. 2021 May;38(5):777-789. doi: 10.1111/echo.15037. Epub 2021 Mar 29. Echocardiography. 2021. PMID: 33778977 Review.
References
-
- Meller C.H., Grinenco S., Aiello H., Córdoba A., Sáenz-Tejeira M.M., Marantz P., Otaño L. Congenital heart disease, prenatal diagnosis and management. Arch. Argent. Pediatr. 2020;118:e149–e161. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

