Real-World Effectiveness and Safety of Selective JAK Inhibitors in Ulcerative Colitis and Crohn's Disease: A Retrospective, Multicentre Study
- PMID: 39768726
- PMCID: PMC11728011
- DOI: 10.3390/jcm13247804
Real-World Effectiveness and Safety of Selective JAK Inhibitors in Ulcerative Colitis and Crohn's Disease: A Retrospective, Multicentre Study
Abstract
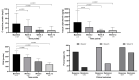
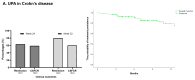
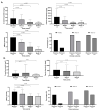
Background/Objectives: Data on the real-world effectiveness and safety of selective JAK inhibitors (JAKis) in ulcerative colitis (UC) and Crohn's disease (CD) are limited. Methods: We conducted a multicentre, retrospective study to assess clinical, biochemical, and endoscopic outcomes of selective JAKis in bio-experienced UC and CD. Results: A total of 246 patients (mean age: 40.5 ± 14.5 years; 131 UC and 115 CD) were included with a median follow-up of 7.5 months. Among the CD patients receiving upadacitinib (n = 115), 76.2% achieved clinical remission (CR) at week 12. Furthermore, 59.5% of the upadacitinib-treated UC patients (n = 100) experienced CR at week 8. Corticosteroid-free CR (CSFCR) was achieved by 76.9% of the CD patients and 80.6% of the UC patients at week 24, while 50.0% and 36.1% experienced endoscopic remission. At week 52, 66.7% of the CD and 86.2% of the UC patients achieved CSFCR, whereas 54.5% and 52.9% had endoscopic remission. In UC, the effectiveness of upadacitinib was not compromised by prior tofacitinib failure, while the upadacitinib-treated CD patients with stricturing and penetrating disease were less likely to achieve CR by the end of the induction phase (p = 0.04). C-reactive protein (p[CD] < 0.0001; p[UC] < 0.0001) and faecal calprotectin (p[CD] < 0.0001; p[UC] = 0.02) decreased significantly in both patient groups as early as week 2. Among the filgotinib-treated UC patients (n = 31), 28.6% were in CR at week 12. At week 24 and 52, 59.1% and 60% achieved CSFCR, while 0.0% and 20.0% had endoscopic remission. Both C-reactive protein (p = 0.04) and faecal calprotectin (p = 0.04) decreased significantly by week 12. Hyperlipidaemia (9.7-9.8%) was the most common adverse event. Conclusions: Selective JAKis are rapidly effective and safe for treating refractory, moderate-to-severe CD and UC.
Keywords: Crohn’s disease; JAK-inhibitors; filgotinib; ulcerative colitis; upadacitinib.
Conflict of interest statement
Talat Bessissow has received honorarium as a speaker or consultant for Abbvie, Alimentiv, BMS, Eli Lilly, Ferring, Fresenius Kabi, Gilead, Janssen, Merck, Pendopharm, Pentax, Pfizer, Roche, Sandoz, Sanofi, Takeda, and Viatris. Jimmy K. Limdi has been a speaker and/or advisory board member for AbbVie, Arena, BioHit, Bristol Myers Squibb, Eli Lilly, Ferring, Galapagos, Janssen, MSD, Pfizer, Takeda, and Tillots and has received research grants from Galapagos and Takeda. Karishma Sethi-Arora has received speaker fees from Celltrion, Galapagos, Janssen, and Takeda. Alessandro Armuzzi received consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, and Takeda; lecture and/or speaker bureau fees from AbbVie, Amgen, Arena, Biogen, Ferring, Galapagos, Gilead, Janssen, MSD, Mitsubishi-Tanabe, Nikkiso, Novartis, Pfizer, Sandoz, Samsung Bioepis, and Takeda; and research grants from MSD, Pfizer, Takeda, and Biogen. Milan Lukas has been a speaker for Abbvie, Takeda, Celltrion, Biogen, and Janssen Cilag. George Michalopoulos has received speaker’s honoraria from MSD, TAKEDA, Jannsen, Amgen, and Abbvie. Elena Chashkova has served as speaker for AbbVie, Janssen, and Takeda. Edoardo V. Savarino has served as speaker for Abbvie, Agave, AGPharma, Alfasigma, Aurora Pharma, CaDiGroup, Celltrion, Dr Falk, EG Stada Group, Fenix Pharma, Fresenius Kabi, Galapagos, Janssen, JB Pharmaceuticals, Innovamedica/Adacyte, Malesci, Mayoly Biohealth, Omega Pharma, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Tillots, and Unifarco; has served as consultant for Abbvie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, Diadema Farmaceutici, Dr. Falk, Fenix Pharma, Fresenius Kabi, Janssen, JB Pharmaceuticals, Merck & Co, Nestlè, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Synformulas GmbH, Takeda, and Unifarco; and he received research support from Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, and Zeta Farmaceutici. Fabiana Castiglione has received speaker’s honoraria from Pfizer, AbbVie, Janssen, Galapagos, Alfasigma, Takeda, and Biogen. Simone Saibeni declares the following paid consultancies and lecture fees: AbbVie, Arena, Ferring, Galapagos, Gilead, Janssen, MSD, Pfizer, and Takeda. Željko Krznarić has received speaker’s honoraria from AbbVie, Janssen, Ferring, Takeda, Pfizer, Sandoz, and Fresenius. Tamás Molnár has received speaker’s honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer, Janssen, Sandoz, MundiPharma, Phytotec, Roche, Fresenius, Bristol-Myers Squibb, Lilly, and Teva. Peter L. Lakatos has been a speaker and/or advisory board member for AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Fresenius Kabi, Gilead, Janssen, Eli Lilly, Merck, Organon, Pendopharm, Pfizer, Roche, Sandoz, and Takeda and has received unrestricted research grants from AbbVie, Gilead, and Pfizer. Klaudia Farkas has received speaker’s honoraria from AbbVie, Janssen, Ferring, Takeda, Pfizer, Bristol-Myers Squibb, and Goodwill Pharma. The other authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures



References
-
- Attauabi M., Dahl E.K., Burisch J., Gubatan J., Nielsen O.H., Seidelin J.B. Comparative onset of effect of biologics and small molecules in moderate-to-severe ulcerative colitis: A systematic review and network meta-analysis. eClinicalMedicine. 2023;57:101866. doi: 10.1016/j.eclinm.2023.101866. - DOI - PMC - PubMed
-
- Attauabi M., Steenholdt C., Poulsen A., Gubatan J., Burisch J., Nielsen O.H., Seidelin J.B. Network meta-analysis: Comparative onset of early effect of biologics and small molecules in moderately to severely active luminal Crohn’s disease. Aliment. Pharmacol. Ther. 2024;60:124–143. doi: 10.1111/apt.18110. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

