Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides
- PMID: 39769004
- PMCID: PMC11676002
- DOI: 10.3390/ijms252413240
Sequence-Specific Free Energy Changes in DNA/RNA Induced by a Single LNA-T Modification in Antisense Oligonucleotides
Abstract
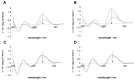
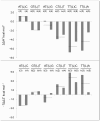
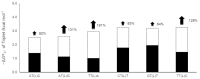
2',4'-methylene bridged nucleic acid/locked nucleic acid (2',4'-BNA/LNA; LNA) is a modified nucleic acid that improves the function of antisense oligonucleotide therapeutics. In particular, LNA in the DNA strand increases its binding affinity for the target RNA. Predicting the binding affinities of LNA-containing antisense oligonucleotides and RNA duplexes is useful for designing antisense oligonucleotides. The nearest neighbor parameters may be useful for binding affinity prediction, similar to those for natural nucleic acids. However, the sequence dependence of the thermodynamic stability of DNA/RNA duplexes containing LNA remains unexplored. Therefore, in this study, we evaluated the thermodynamic stabilities of DNA/RNA duplexes containing a single LNA modification in the DNA strand. We found that LNA-stabilized DNA/RNA duplexes averaged -1.5 kcal mol-1. Our findings suggest that the thermodynamic stabilization effect of LNA is sequence-specific.
Keywords: LNA-containing antisense oligonucleotides; locked nucleic acid; thermodynamic stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kawasaki A.M., Casper M.D., Freier S.M., Lesnik E.A., Zounes M.C., Cummins L.L., Gonzalez C., Cook P.D. Uniformly modified 2’-deoxy-2’-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J. Med. Chem. 1993;36:831–841. doi: 10.1021/jm00059a007. - DOI - PubMed
-
- Obika S., Nanbu D., Hari Y., Morio K., In Y., Ishida T., Imanishi T. Synthesis of 2’-O,4’-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3’-endo sugar puckering. Tetrahedron Lett. 1997;38:8735–8738. doi: 10.1016/S0040-4039(97)10322-7. - DOI
-
- Singh S.K., Nielsen P., Koshkin A.A., Wengel J. LNA (locked nucleic acids): Synthesis and high-affinity nucleic acid recognition. Chem. Commun. 1998;4:455–456. doi: 10.1039/a708608c. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

