Extracellular Vesicle miR-122-5p as a Prognostic Biomarker in Pediatric Classical Hodgkin Lymphoma
- PMID: 39769007
- PMCID: PMC11678363
- DOI: 10.3390/ijms252413243
Extracellular Vesicle miR-122-5p as a Prognostic Biomarker in Pediatric Classical Hodgkin Lymphoma
Abstract
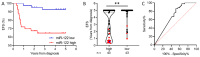
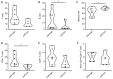
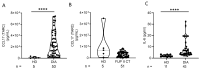
Currently, risk stratification for pediatric Hodgkin lymphoma is based on clinical factors such as stage, bulk, and systemic symptoms. Novel minimally invasive biomarkers could enhance both prognosis and treatment strategies. Therefore, the plasma extracellular vesicles' microRNA profile was characterized by small RNA sequencing in 36 classical Hodgkin lymphoma cases and these findings were confirmed in an extended cohort of 86 patients by RT-qPCR. It was found that the levels of miR-122-5p at diagnosis were significantly higher (p-value: 0.0002) in patients who relapsed compared to patients in remission. The 5-year event-free survival of cases with high and low levels of miR-122-5p was 65 ± 7% and 93 ± 4%, respectively. MiR-122-5p levels were significantly associated with clinical events in both univariate (p-value: 0.0009) and multivariate (p-value: 0.0037) analysis (hazard ratio 5.8). Target prediction analysis suggests an involvement in the polarization of immune cells. The phenotypic characterization of peripheral blood mononuclear cells in 12 patients showed significantly increased levels of CD4+ T-cells in cases with high miR-122-5p levels as compared to low levels (p-value: 0.048). Moreover, CCL17 (TARC) and IL-6 plasma levels at diagnosis were significantly higher as compared to healthy donors (p-value: ≤0.0001). MiR-122-5p could complement current prognostic assays to identify patients at high risk of relapse.
Keywords: Hodgkin lymphoma; T-cells; biomarkers; diagnostics; extracellular vesicles; immune escape; inflammation; liquid biopsy; miRNA; relapse.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Tatalovich Z., Mariotto A., Ruhl J., Lewis D.R., et al. SEER Cancer Statistics Review, 1975–2018. National Cancer Institute; Bethesda, MD, USA: 2021.
-
- Schmitz N., Pfistner B., Sextro M., Sieber M., Carella A.M., Haenel M., Boissevain F., Zschaber R., Müller P., Kirchner H., et al. Aggressive Conventional Chemotherapy Compared with High-Dose Chemotherapy with Autologous Haemopoietic Stem-Cell Transplantation for Relapsed Chemosensitive Hodgkin’s Disease: A Randomised Trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. - DOI - PubMed
-
- Moskowitz C.H., Nimer S.D., Zelenetz A.D., Trippett T., Hedrick E.E., Filippa D.A., Louie D., Gonzales M., Walits J., Coady-Lyons N., et al. A 2-Step Comprehensive High-Dose Chemoradiotherapy Second-Line Program for Relapsed and Refractory Hodgkin Disease: Analysis by Intent to Treat and Development of a Prognostic Model. Blood. 2001;97:616–623. doi: 10.1182/blood.V97.3.616. - DOI - PubMed
-
- Chen R., Zinzani P.L., Fanale M.A., Armand P., Johnson N.A., Brice P., Radford J., Ribrag V., Molin D., Vassilakopoulos T.P., et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 101072735/European Union´s Horizon Europe Marie Skłodowska-Curie Actions Doctoral Networks
- IG 2017 #20052/Comitato Assistenza Socio-sanitaria in Oncoematologia Pediatrica
- IG #2023 #28966/Associazione italiana contro le leucemie-linfomi e mieloma
- CN00000041 CN3 Spoke #6 "RNA chemistry"/MUR PNRR "National Center for Gene Therapy and Drugs based on RNA Technology"
- Project no. CN00000013 CN1 Spoke #8 "In Silico Medicine & Omics Data"/"National Center for HPC, Big Data and Quantum Computing"
LinkOut - more resources
Full Text Sources
Medical
Research Materials

