Altered Cellular Metabolism Is a Consequence of Loss of the Ataxia-Linked Protein Sacsin
- PMID: 39769008
- PMCID: PMC11675909
- DOI: 10.3390/ijms252413242
Altered Cellular Metabolism Is a Consequence of Loss of the Ataxia-Linked Protein Sacsin
Abstract
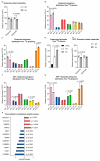
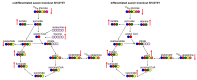
Mitochondrial dysfunction is implicated in the pathogenesis of the neurological condition autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS), yet precisely how the mitochondrial metabolism is affected is unknown. Thus, to better understand changes in the mitochondrial metabolism caused by loss of the sacsin protein (encoded by the SACS gene, which is mutated in ARSACS), we performed mass spectrometry-based tracer analysis, with both glucose- and glutamine-traced carbon. Comparing the metabolite profiles between wild-type and sacsin-knockout cell lines revealed increased reliance on aerobic glycolysis in sacsin-deficient cells, as evidenced by the increase in lactate and reduction of glucose. Moreover, sacsin knockout cells differentiated towards a neuronal phenotype had increased levels of tricarboxylic acid cycle metabolites relative to the controls. We also observed disruption in the glutaminolysis pathway in differentiated and undifferentiated cells in the absence of sacsin. In conclusion, this work demonstrates consequences for cellular metabolism associated with a loss of sacsin, which may be relevant to ARSACS.
Keywords: ARSACS; aerobic glycolysis; metabolism; mitochondria; sacsin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Engert J.C., Bérubé P., Mercier J., Doré C., Lepage P., Ge B., Bouchard J.-P., Mathieu J., Melançon S.B., Schalling M., et al. ARSACS, a spastic ataxia common in northeastern Québec, is caused by mutations in a new gene encoding an 11.5-kb ORF. Nat. Genet. 2000;24:120–125. doi: 10.1038/72769. - DOI - PubMed
-
- Parfitt D.A., Michael G.J., Vermeulen E.G., Prodromou N.V., Webb T.R., Gallo J.-M., Cheetham M.E., Nicoll W.S., Blatch G.L., Chapple J.P. The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum. Mol. Genet. 2009;18:1556–1565. doi: 10.1093/hmg/ddp067. - DOI - PMC - PubMed
-
- Bradshaw T.Y., Romano L.E.L., Duncan E.J., Nethisinghe S., Abeti R., Michael G.J., Giunti P., Vermeer S., Chapple J.P. A reduction in Drp1-mediated fission compromises mitochondrial health in autosomal recessive spastic ataxia of Charlevoix Saguenay. Hum. Mol. Genet. 2016;25:3232–3244. doi: 10.1093/hmg/ddw173. - DOI - PMC - PubMed
-
- Girard M., Larivière R., Parfitt D.A., Deane E.C., Gaudet R., Nossova N., Blondeau F., Prenosil G., Vermeulen E.G.M., Duchen M.R., et al. Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) Proc. Natl. Acad. Sci. USA. 2012;109:1661–1666. doi: 10.1073/pnas.1113166109. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

