Enhancing Clinical Applications by Evaluation of Sensitivity and Specificity in Whole Exome Sequencing
- PMID: 39769013
- PMCID: PMC11678496
- DOI: 10.3390/ijms252413250
Enhancing Clinical Applications by Evaluation of Sensitivity and Specificity in Whole Exome Sequencing
Abstract
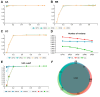
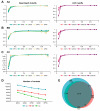
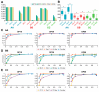
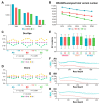
The cost-effectiveness of whole exome sequencing (WES) remains controversial due to variant call variability, necessitating sensitivity and specificity evaluation. WES was performed by three companies (AA, BB, and CC) using reference standards composed of DNA from hydatidiform mole and individual blood at various ratios. Sensitivity was assessed by the detection rate of null-homozygote (N-H) alleles at expected variant allelic fractions, while false positive (FP) errors were counted for unexpected alleles. Sensitivity was approximately 20% for in-house results from BB and CC and around 5% for AA. Dynamic Read Analysis for GENomics (DRAGEN) analyses identified 1.34 to 1.71 times more variants, detecting over 96% of in-house variants, with sensitivity for common variants increasing to 5%. In-house FP errors varied significantly among companies (up to 13.97 times), while DRAGEN minimized this variation. Despite DRAGEN showing higher FP errors for BB and CC, the increased sensitivity highlights the importance of effective bioinformatic conditions. We also assessed the potential effects of target enrichment and proposed optimal cutoff values for the read depth and variant allele fraction in WES. Optimizing bioinformatic analysis based on sensitivity and specificity from reference standards can enhance variant detection and improve the clinical utility of WES.
Keywords: WES; cancer; false negative error; false positive error; mutation; quality control; reference standard.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

