Directed Mutagenesis for Arginine Substitution of a Phaseolus acutifolius Recombinant Lectin Disrupts Its Cytotoxic Activity
- PMID: 39769023
- PMCID: PMC11676905
- DOI: 10.3390/ijms252413258
Directed Mutagenesis for Arginine Substitution of a Phaseolus acutifolius Recombinant Lectin Disrupts Its Cytotoxic Activity
Abstract
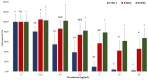
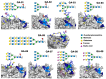
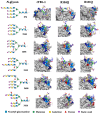
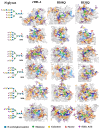
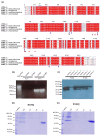
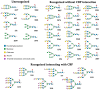
Recently, we reported that a recombinant Tepary bean (Phaseolus acutifolius) lectin (rTBL-1) induces apoptosis in colon cancer cell lines and that cytotoxicity was related to differential recognition of β1-6 branched N-glycans. Sequencing analysis and resolution of the rTBL-1 3D structure suggest that glycan specificity could be strongly influenced by two arginine residues, R103 and R130, located in the carbohydrate binding pocket. The aim of this work was to determine the contribution of these residues towards cytotoxic activity. Two rTBL-1 mutants were produced in Pichia pastoris, biochemically characterized, and cytotoxic effects were evaluated on human colorectal cancer cells (HT-29). Substitution of either of the arginine residues with glutamines resulted in significant reductions in cytotoxic activity, with losses of 1.5 and 3 times for R103 and R130, respectively. Docking analysis showed that the mutations decreased lectin affinity binding to some Epidermal Growth Factor Receptor (EGFR)-related N-glycans. Together, these findings confirm that both of the selected arginine residues (R103 and R130) play a key role in the recognition of tumor cell glycoconjugates by rTBL-1.
Keywords: branched N-glycans; cancer; recombinant lectins; tepary bean.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) with Specific Recognition for Cancer-Associated Glycans: Production, Structural Characterization, and Target Identification.Biomolecules. 2020 Apr 23;10(4):654. doi: 10.3390/biom10040654. Biomolecules. 2020. PMID: 32340396 Free PMC article.
-
Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay.Int J Mol Sci. 2021 Jan 21;22(3):1049. doi: 10.3390/ijms22031049. Int J Mol Sci. 2021. PMID: 33494324 Free PMC article.
-
EGFR and p38MAPK Contribute to the Apoptotic Effect of the Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) in Colon Cancer Cells.Pharmaceuticals (Basel). 2023 Feb 14;16(2):290. doi: 10.3390/ph16020290. Pharmaceuticals (Basel). 2023. PMID: 37259433 Free PMC article.
-
Tepary bean (Phaseolus acutifolius).Methods Mol Biol. 2006;343:407-14. doi: 10.1385/1-59745-130-4:407. Methods Mol Biol. 2006. PMID: 16988363 Review.
-
Lectin engineering, a molecular evolutionary approach to expanding the lectin utilities.Molecules. 2015 Apr 27;20(5):7637-56. doi: 10.3390/molecules20057637. Molecules. 2015. PMID: 25923514 Free PMC article. Review.
Cited by
-
Phaseolus acutifolius Recombinant Lectin Exerts Differential Proapoptotic Activity on EGFR+ and EGFR- Colon Cancer Cells and Provokes T Cell-Assisted Antitumor Responses in Mice.Pharmaceuticals (Basel). 2025 Feb 5;18(2):213. doi: 10.3390/ph18020213. Pharmaceuticals (Basel). 2025. PMID: 40006027 Free PMC article.
-
Special Issue: Discovery of Bioactive Phytochemicals' Molecular Mechanisms Against Different Diseases Based on Network Pharmacology and Molecular Docking.Int J Mol Sci. 2025 May 9;26(10):4516. doi: 10.3390/ijms26104516. Int J Mol Sci. 2025. PMID: 40429659 Free PMC article.
References
-
- Wang P., Leng X., Duan J., Zhu Y., Wang J., Yan Z., Min S., Wei D., Wang X. Functional Component Isolated from Phaseolus vulgaris Lectin Exerts In Vitro and In Vivo Anti-Tumor Activity Through Potentiation of Apoptosis and Immunomodulation. Molecules. 2021;26:498. doi: 10.3390/molecules26020498. - DOI - PMC - PubMed
-
- Kiss R., Camby I., Duckworth C., De Decker R., Salmon I., Pasteels J.L., Danguy A., Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, concanavalin A, wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut. 1997;40:253–261. doi: 10.1136/gut.40.2.253. - DOI - PMC - PubMed
-
- Bardocz S., Grant G., Duguid T.J., Brown D.S., Pusztai A., Pryme I.F. Intracellular levels of polyamines in Krebs II lymphosarcoma cells in mice fed phytohaemagglutinin-containing diets are coupled with altered tumour growth. Cancer Lett. 1997;121:25–29. doi: 10.1016/S0304-3835(97)00316-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

