MicroRNA-Based Liquid Biopsy for Cervical Cancer Diagnostics and Treatment Monitoring
- PMID: 39769036
- PMCID: PMC11678179
- DOI: 10.3390/ijms252413271
MicroRNA-Based Liquid Biopsy for Cervical Cancer Diagnostics and Treatment Monitoring
Abstract
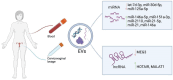
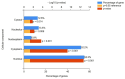
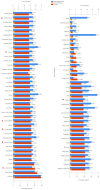
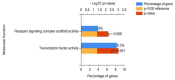
Despite prevention strategies, cervical cancer remains a significant public health issue. Human papillomavirus plays a critical role in its development, and early detection is vital to improve patient outcomes. The incidence of cervical cancer is projected to rise, necessitating better diagnostic tools. Traditional screening methods like the cytological examination and human papillomavirus testing have limitations in sensitivity and reproducibility. Liquid-based cytology offers some improvements, but the need for more reliable and sensitive techniques persists, particularly for detecting precancerous lesions. Liquid biopsy is a non-invasive method that analyzes cancer-derived products in biofluids like blood, offering potential for real-time monitoring of tumor progression, metastasis, and treatment response. It can be based on detection of circulating tumor cells (CTCs), circulating free DNA (cfDNA), and microRNAs (miRNAs). This review particularly underlines the potential of microRNAs, which are transported by extracellular vesicles. Overall, this article underscores the importance of continued research into non-invasive diagnostic methods like liquid biopsy to enhance cervical cancer screening and treatment monitoring.
Keywords: biomarkers; cervical cancer; diagnosis; exosomes; extracellular vesicles; miRNA; microRNA; treatment monitoring.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Singh D., Vignat J., Lorenzoni V., Eslahi M., Ginsburg O., Lauby-Secretan B., Arbyn M., Basu P., Bray F., Vaccarella S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health. 2023;11:e197–e206. doi: 10.1016/S2214-109X(22)00501-0. - DOI - PMC - PubMed
-
- Gates A., Pillay J., Reynolds D., Stirling R., Traversy G., Korownyk C., Moore A., Thériault G., Thombs B.D., Little J., et al. Screening for the prevention and early detection of cervical cancer: Protocol for systematic reviews to inform Canadian recommendations. Syst. Rev. 2021;10:2. doi: 10.1186/s13643-020-01538-9. - DOI - PMC - PubMed
-
- World Health Organization . Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. World Health Organization; Geneva, Switzerland: 2020. 52p

