Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum
- PMID: 39769074
- PMCID: PMC11678653
- DOI: 10.3390/ijms252413309
Increased Levels of hsa-miR-199a-3p and hsa-miR-382-5p in Maternal and Neonatal Blood Plasma in the Case of Placenta Accreta Spectrum
Abstract
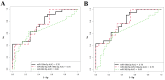
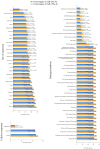
Despite the increasing number of placenta accreta spectrum (PAS) cases in recent years, its impact on neonatal outcomes and respiratory morbidity, as well as the underlying pathogenetic mechanism, has not yet been extensively studied. Moreover, no study has yet demonstrated the effectiveness of antenatal corticosteroid therapy (CT) for the prevention of respiratory distress syndrome (RDS) in newborns of mothers with PAS at the molecular level. In this regard, microRNA (miRNA) profiling by small RNA deep sequencing and quantitative real-time PCR was performed on 160 blood plasma samples from preterm infants (gestational age: 33-36 weeks) and their mothers who had been diagnosed with or without PAS depending on the timing of the antenatal RDS prophylaxis. A significant increase in hsa-miR-199a-3p and hsa-miR-382-5p levels was observed in the blood plasma of the newborns from mothers with PAS compared to the control group. A clear trend toward the normalization of hsa-miR-199a-3p and hsa-miR-382-5p levels in the neonatal blood plasma of the PAS groups was observed when CT was administered within 14 days before delivery, but not beyond 14 days. Direct correlations were found among the hsa-miR-382-5p level in neonatal blood plasma and the hsa-miR-199a-3p level in the same sample (r = 0.49; p < 0.001), the oxygen requirements in the NICU (r = 0.41; p = 0.001), the duration of the NICU stay (r = 0.31; p = 0.019), and the severity of the newborn's condition based on the NEOMOD scale (r = 0.36; p = 0.005). Logistic regression models based on the maternal plasma levels of hsa-miR-199a-3p and hsa-miR-382-5p predicted the need for cardiotonic therapy, invasive mechanical ventilation, or high-frequency oscillatory ventilation in newborns during the early neonatal period, with a sensitivity of 95-100%. According to the literary data, these miRNAs regulate fetal organogenesis via IGF-1, the formation of proper lung tissue architecture, surfactant synthesis in alveolar cells, and vascular tone.
Keywords: PCR; RDS; antenatal corticosteroid therapy; blood plasma; deep sequencing; miRNA; neonatal complication; placenta accreta spectrum.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
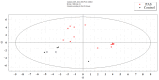

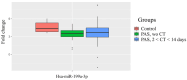
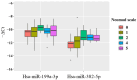
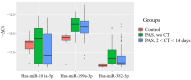
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

