Sigma-1 Receptor as a Novel Therapeutic Target in Diabetic Kidney Disease
- PMID: 39769092
- PMCID: PMC11679586
- DOI: 10.3390/ijms252413327
Sigma-1 Receptor as a Novel Therapeutic Target in Diabetic Kidney Disease
Abstract
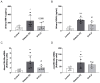
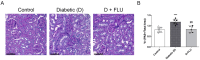
Diabetic kidney disease (DKD) is the leading cause of chronic kidney disease. Current treatments for DKD do not halt renal injury progression, highlighting an urgent need for therapies targeting key disease mechanisms. Our previous studies demonstrated that activating the Sigma-1 receptor (S1R) with fluvoxamine (FLU) protects against acute kidney injury by inhibiting inflammation and ameliorating the effect of hypoxia. Based on these, we hypothesized that FLU might exert a similar protective effect in DKD. Diabetes was induced in male Wistar rats using streptozotocin, followed by a seven-week FLU treatment. Metabolic and renal parameters were assessed along with a histological analysis of glomerular damage and fibrosis. The effects of FLU on inflammation, hypoxia, and fibrosis were tested in human proximal tubular cells and normal rat kidney fibroblasts. FLU improved renal function and reduced glomerular damage and tubulointerstitial fibrosis. It also mitigated inflammation by reducing TLR4, IL6, and NFKB1 expressions and moderated the cellular response to tubular hypoxia. Additionally, FLU suppressed TGF-β1-induced fibrotic processes and fibroblast transformation. These findings suggest that S1R activation can slow DKD progression and protect renal function by modulating critical inflammatory, hypoxic, and fibrotic pathways; therefore, it might serve as a promising novel drug target for preventing DKD.
Keywords: Sigma-1 receptor; diabetic kidney disease; fibrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

