TRAPPopathies: Severe Multisystem Disorders Caused by Variants in Genes of the Transport Protein Particle (TRAPP) Complexes
- PMID: 39769094
- PMCID: PMC11728246
- DOI: 10.3390/ijms252413329
TRAPPopathies: Severe Multisystem Disorders Caused by Variants in Genes of the Transport Protein Particle (TRAPP) Complexes
Abstract
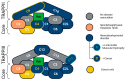
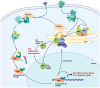
The TRAPP (TRAnsport Protein Particle) protein complex is a multi-subunit complex involved in vesicular transport between intracellular compartments. The TRAPP complex plays an important role in endoplasmic reticulum-to-Golgi and Golgi-to-plasma membrane transport, as well as autophagy. TRAPP complexes comprise a core complex, TRAPPI, and the association of peripheral protein subunits to make two complexes, known as TRAPPII and TRAPPIII, which act as Guanine Nucleotide Exchange Factors (GEFs) of Rab11 and Rab1, respectively. Rab1 and Rab11 are GTPases that mediate cargo selection, packaging, and delivery during pre- and post-Golgi transport in the secretory pathway. Rab1 is also required for the first step of macroautophagy, a cellular recycling pathway. Pathogenic variants in genes encoding protein subunits of the TRAPP complex are associated with a range of rare but severe neurological, skeletal, and muscular disorders, collectively called TRAPPopathies. Disease-causing variants have been identified in multiple subunits of the TRAPP complex; however, little is known about the underlying disease mechanisms. In this review, we will provide an overview of the current knowledge surrounding disease-associated variants of the TRAPP complex subunits, propose new insights into the underlying disease pathology, and suggest future research directions into the underlying disease mechanisms.
Keywords: Rab GTPase; TRAPP complex; autophagy; human genetics; intracellular trafficking; neurological disease; pediatrics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- NA/mRNA Victoria Pilot
- NA/VESKI Near Miss Pilot Award
- NA/The Royal Children's Hospital Foundation
- MRF2015957/Medical Research Future Fund (MRFF) Stem cell therapies mission grant
- NA/Therapeutic Innovation Australia
- NA/State Government of Victoria's Operational Infrastructure Support Program
- NNF21CC0073729/The Novo Nordisk Foundation Center for Stem Cell Medicine, reNEW, funded by the Novo Nordisk Foundation grant
- R21 NS125790/NS/NINDS NIH HHS/United States
- R35 GM141479/GM/NIGMS NIH HHS/United States
- NA/Australian Society for Inborn Errors in Metabolism
- NA/RTW Charitable Foundation
LinkOut - more resources
Full Text Sources

