The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment
- PMID: 39769097
- PMCID: PMC11727763
- DOI: 10.3390/ijms252413334
The Emerging Scenario of Ferroptosis in Pancreatic Cancer Tumorigenesis and Treatment
Abstract
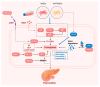
Pancreatic cancer remains one of the most lethal forms of cancer. Currently, there is a lack of effective drug treatments for pancreatic cancer. However, as a newly discovered form of non-apoptotic cell death, ferroptosis has garnered increasing attention in relation to pancreatic cancer. Understanding the role of ferroptosis in the tumorigenesis and treatment of pancreatic cancer may enable more effective clinical trials and treatments for pancreatic cancer and may minimize side effects or restrict the emergence of drug resistance. In this review, we summarize the current knowledge regarding the process and underlying mechanisms of ferroptosis, as well as its dual role in both promoting tumorigenesis and facilitating treatment strategies for pancreatic cancer. Additionally, how ferroptosis is implicated in the development of pancreatitis and insulin resistance, indicating that ferroptosis may play an important role in the risk of pancreatitis- and insulin-resistance-related pancreatic cancers, is also addressed.
Keywords: drug resistance; ferroptosis; immunotherapy; pancreatic cancer; tumorigenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Hu C., Hart S.N., Polley E.C., Gnanaolivu R., Shimelis H., Lee K.Y., Lilyquist J., Na J., Moore R., Antwi S.O., et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319:2401–2409. doi: 10.1001/jama.2018.6228. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

