Depletion of Gibberellin Signaling Up-Regulates LBD16 Transcription and Promotes Adventitious Root Formation in Arabidopsis Leaf Explants
- PMID: 39769105
- PMCID: PMC11678481
- DOI: 10.3390/ijms252413340
Depletion of Gibberellin Signaling Up-Regulates LBD16 Transcription and Promotes Adventitious Root Formation in Arabidopsis Leaf Explants
Abstract
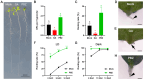
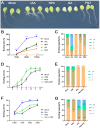
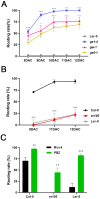
Adventitious root (AR) formation in plants originates from non-root organs such as leaves and hypocotyls. Auxin signaling is essential for AR formation, but the roles of other phytohormones are less clear. In Arabidopsis, at least two distinct mechanisms can produce ARs, either from hypocotyls as part of the general root architecture or from wounded organs during de novo root regeneration (DNRR). In previous reports, gibberellin acid (GA) appeared to play reverse roles in both types of ARs, since GA treatment blocks etiolation-induced AR formation from hypocotyls, whereas GA synthesis and signaling mutants apparently displayed reduced DNRR from detached leaves. In order to clarify this contradiction, we employed the GA biosynthesis inhibitor paclobutrazol (PBZ) and found that PBZ had positive effects on both types of AR formation in Arabidopsis. Consistently, GA treatment had negative effects on both AR formation mechanisms, while loss of GA synthesis and signaling promoted DNRR under our conditions. Our results show that PBZ treatment can rescue declined AR formation in difficult-to-root leaf explants such as erecta receptor mutants. Furthermore, transcriptional profiling revealed that PBZ treatment altered GA, brassinosteroids, and auxin responses, which included the up-regulation of LBD16 that is well known for its pivotal role in AR initiation.
Keywords: LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16); LATERAL ROOT PRIMORDIUM 1 (LRP1); adventitious roots; blocking of gibberellin biosynthesis; de novo root regeneration (DNRR); paclobutrazol (PBZ); rescuing difficult-to-root mutant leaf explants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis.BMC Plant Biol. 2019 Jan 31;19(1):46. doi: 10.1186/s12870-019-1659-4. BMC Plant Biol. 2019. PMID: 30704405 Free PMC article.
-
Functional Analysis of CLE26 in Controlling De Novo Root Regeneration from Detached Arabidopsis Leaves.Int J Mol Sci. 2024 Dec 7;25(23):13156. doi: 10.3390/ijms252313156. Int J Mol Sci. 2024. PMID: 39684866 Free PMC article.
-
The epigenetic regulator ULTRAPETALA1 suppresses de novo root regeneration from Arabidopsis leaf explants.Plant Signal Behav. 2022 Dec 31;17(1):2031784. doi: 10.1080/15592324.2022.2031784. Epub 2022 Feb 15. Plant Signal Behav. 2022. PMID: 35164655 Free PMC article.
-
De novo root regeneration from leaf explant: a mechanistic review of key factors behind cell fate transition.Planta. 2025 Jan 14;261(2):33. doi: 10.1007/s00425-025-04616-1. Planta. 2025. PMID: 39808280 Review.
-
Pivotal role of LBD16 in root and root-like organ initiation.Cell Mol Life Sci. 2018 Sep;75(18):3329-3338. doi: 10.1007/s00018-018-2861-5. Epub 2018 Jun 25. Cell Mol Life Sci. 2018. PMID: 29943076 Free PMC article. Review.
References
-
- Taramino G., Sauer M., Stauffer J.L., Multani D., Niu X., Sakai H., Hochholdinger F. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007;50:649–659. doi: 10.1111/j.1365-313X.2007.03075.x. - DOI - PubMed
-
- Xu C., Tai H., Saleem M., Ludwig Y., Majer C., Berendzen K.W., Nagel K.A., Wojciechowski T., Meeley R.B., Taramino G., et al. Cooperative action of the paralogous maize lateral organ boundaries (LOB) domain proteins RTCS and RTCL in shoot-borne root formation. New Phytol. 2015;207:1123–1133. doi: 10.1111/nph.13420. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

