Caryophyllene Oxide, a Bicyclic Terpenoid Isolated from Annona macroprophyllata with Antitumor Activity: In Vivo, In Vitro, and In Silico Studies
- PMID: 39769118
- PMCID: PMC11676109
- DOI: 10.3390/ijms252413355
Caryophyllene Oxide, a Bicyclic Terpenoid Isolated from Annona macroprophyllata with Antitumor Activity: In Vivo, In Vitro, and In Silico Studies
Abstract
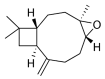
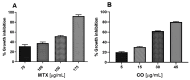
The Annona genus contains some species used in Mexican traditional medicine for the treatment cancer, including Annona macroprophyllata (A. macroprophyllata). The present study aimed to investigate the anticancer activity of caryophyllene oxide (CO) isolated from A. macroprophyllata using in vivo, in vitro, and in silico approaches. The identification of CO was performed using gas chromatography-mass spectroscopy and NMR methods. Antilymphoma activity was evaluated in male and female Balb/c mice inoculated with U-937 cells. Cytotoxic activity was evaluated using the WST method and flow cytometry was used to determine the type of cell death. Acute oral toxicity was determined, and a molecular docking study was performed using target proteins associated with cancer, including, HMG-CoA, Bcl-2, Mcl-1, and VEGFR-2. Results showed that CO exhibited significant antilymphoma and cytotoxic activities, and its effects were comparable to MTX. In addition, flow cytometry showed that the anticancer activity of CO could be mediated by the induction of late apoptosis and necrosis. The result for the acute oral toxicity of CO was classified in category 4, suggesting it is low risk. Finally, molecular coupling studies showed that CO had more affinity for the enzymes HMG-CoA reductase and Bcl-2. Our study provides evidences that CO is a potential anticancer agent for the treatment of histiocytic lymphoma.
Keywords: Annona macroprophyllata; U-937 cells; acute oral toxicity; antilymphoma activity; caryophyllene oxide; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


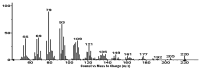



Similar articles
-
Understanding the Antilymphoma Activity of Annona macroprophyllata Donn and Its Acyclic Terpenoids: In Vivo, In Vitro, and In Silico Studies.Molecules. 2022 Oct 21;27(20):7123. doi: 10.3390/molecules27207123. Molecules. 2022. PMID: 36296714 Free PMC article.
-
In Vivo, In Vitro and In Silico Anticancer Activity of Ilama Leaves: An Edible and Medicinal Plant in Mexico.Molecules. 2024 Apr 24;29(9):1956. doi: 10.3390/molecules29091956. Molecules. 2024. PMID: 38731446 Free PMC article.
-
Antitumor Potential of Annona muricata Linn. An Edible and Medicinal Plant in Mexico: In Vitro, In Vivo, and Toxicological Studies.Molecules. 2021 Dec 18;26(24):7675. doi: 10.3390/molecules26247675. Molecules. 2021. PMID: 34946755 Free PMC article.
-
Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities.Molecules. 2019 Dec 3;24(23):4419. doi: 10.3390/molecules24234419. Molecules. 2019. PMID: 31816948 Free PMC article. Review.
-
β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties.Cancer Med. 2016 Oct;5(10):3007-3017. doi: 10.1002/cam4.816. Epub 2016 Sep 30. Cancer Med. 2016. PMID: 27696789 Free PMC article. Review.
Cited by
-
The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer.Molecules. 2025 Apr 14;30(8):1751. doi: 10.3390/molecules30081751. Molecules. 2025. PMID: 40333803 Free PMC article. Review.
-
Immunomodulatory Potential of Kaempferol Isolated from Peronema canescens Jack. Leaves Through Inhibition of IL-6 Expression.Int J Mol Sci. 2025 Mar 27;26(7):3068. doi: 10.3390/ijms26073068. Int J Mol Sci. 2025. PMID: 40243778 Free PMC article.
References
-
- Alonso-Castro A.J., Villarreal M.L., Salazar-Olivo L.A., Gomez-Sanchez M., Dominguez F., Garcia-Carranca A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011;133:945–972. doi: 10.1016/j.jep.2010.11.055. - DOI - PubMed
-
- Secretaria del Medio Ambiente y Recursos Naturales. 2021. [(accessed on 22 July 2024)]. Available online: https://www.gob.mx/semarnat/articulos/plantas-medicinales-de-mexico?idio....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

