Human Liver MSCs Retain Their Basic Cellular Properties in Chronically Inflamed Liver Tissue
- PMID: 39769138
- PMCID: PMC11676302
- DOI: 10.3390/ijms252413374
Human Liver MSCs Retain Their Basic Cellular Properties in Chronically Inflamed Liver Tissue
Abstract
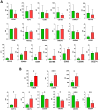
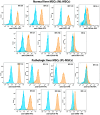
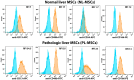
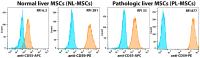
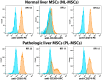
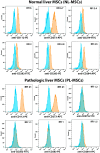
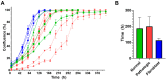
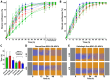
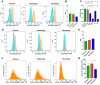
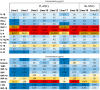
Every 25th death worldwide is associated with liver pathology. The development of novel approaches to liver diseases therapy and protocols for maintaining the vital functions of patients on the liver transplant waiting list are urgently needed. Resident mesenchymal stem cells (MSCs) play a significant role in supporting liver tissue integrity and improve the liver condition after infusion. However, it remains unclear whether MSCs isolated from chronically inflamed livers are similar in their basic cellular properties to MSCs obtained from healthy livers. We applied a large array of tests to compare resident MSCs isolated from apparently normal liver tissue and from chronically inflamed livers of patients with fibrosis, cirrhosis, and viral hepatitis. Chronic inflammatory environment did not alter the major cellular characteristics of MSCs, including the expression of MSC markers, stem cell markers, adhesion molecules, and the hallmarks of senescence, as well as cell proliferation, migration, and secretome. Only the expression of some immune checkpoints and toll-like receptors was different. Evidently, MSCs with unchanged cellular properties are present in human liver even at late stages of inflammatory diseases. These cells can be isolated and used as starting material in the development of cell therapies of liver diseases.
Keywords: cell phenotype; cell therapy; cellular senescence; cirrhosis; fibrosis; liver mesenchymal stem cell; migration; proliferation; secretome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lin B.L., Chen J.F., Qiu W.H., Wang K.W., Xie D.Y., Chen X.Y., Liu Q.L., Peng L., Li J.G., Mei Y.Y., et al. Allogeneic Bone Marrow–Derived Mesenchymal Stromal Cells for Hepatitis B Virus–Related Acute-on-Chronic Liver Failure: A Randomized Controlled Trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. - DOI - PubMed
-
- Shi M., Li Y.Y., Xu R.N., Meng F.P., Yu S.J., Fu J.L., Hu J.H., Li J.X., Wang L.F., Jin L., et al. Mesenchymal Stem Cell Therapy in Decompensated Liver Cirrhosis: A Long-Term Follow-up Analysis of the Randomized Controlled Clinical Trial. Hepatol. Int. 2021;15:1431–1441. doi: 10.1007/s12072-021-10199-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

