How Tumors Affect Hemodynamics: A Diffusion Study on the Zebrafish Transplantable Model of Medullary Thyroid Carcinoma by Selective Plane Illumination Microscopy
- PMID: 39769158
- PMCID: PMC11678154
- DOI: 10.3390/ijms252413392
How Tumors Affect Hemodynamics: A Diffusion Study on the Zebrafish Transplantable Model of Medullary Thyroid Carcinoma by Selective Plane Illumination Microscopy
Abstract
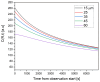
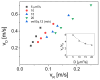
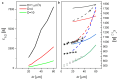
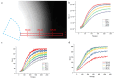
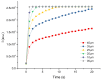
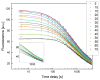
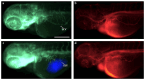
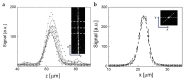
Medullary thyroid carcinoma (MTC), a rare neuroendocrine tumor comprising 3-5% of thyroid cancers, arises from calcitonin-producing parafollicular C cells. Despite aggressive behavior, surgery remains the primary curative treatment, with limited efficacy reported for radiotherapy and chemotherapy. Recent efforts have explored the pathogenetic mechanisms of MTC, identifying it as a highly vascularized neoplasm overexpressing pro-angiogenic factors. Building on the established benefits of zebrafish embryos, we previously created an in vivo MTC xenograft platform that allows real-time observation of tumor-induced angiogenesis and evaluation of the anti-angiogenic effects of tyrosine kinase inhibitors. In this study, we present a method using selective plane illumination microscopy (SPIM) to characterize vascular permeability in these xenografted embryos. Taking advantage of dextran injections into the blood flow of zebrafish embryos, we found that the diffusion coefficient in embryos grafted with MTC cells was about tenfold lower compared with the same parameter in controls. The results demonstrate the potential of our approach to estimate diffusion parameters, providing valuable insights into vascular permeability changes in MTC-implanted zebrafish embryos compared with controls. Our study sheds light on the intricate vascular biology of MTC, offering a promising tool for future investigations into tumor-induced angiogenesis and therapeutic strategies in diverse neoplasms.
Keywords: medullary thyroid carcinoma (MTC); selective plane illumination microscopy (SPIM); tumor xenograft; vascular permeability; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Modigliani E., Cohen R., Campos J.M., Conte-Devolx B., Maes B., Boneu A., Schlumberger M., Bigorgne J.C., Dumontier P., Leclerc L., et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: Results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin. Endocrinol. 1998;48:265–273. doi: 10.1046/j.1365-2265.1998.00392.x. - DOI - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

