The N-Terminal Mutations of cMyBP-C Affect Calcium Regulation, Kinetics, and Force of Muscle Contraction
- PMID: 39769170
- PMCID: PMC11677233
- DOI: 10.3390/ijms252413405
The N-Terminal Mutations of cMyBP-C Affect Calcium Regulation, Kinetics, and Force of Muscle Contraction
Abstract
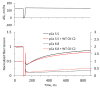
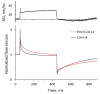
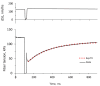
The cardiac myosin binding protein-C (cMyBP-C) regulates cross-bridge formation and controls the duration of systole and diastole at the whole heart level. As known, mutations in cMyBP-C increase the cross-bridge number and rate of their cycling, hypercontractility, and myocardial hypertrophy. We investigated the effects of the mutations D75N and P161S of cMyBP-C related to hypertrophic cardiomyopathy on the mechanism of force generation in isolated slow skeletal muscle fibers. The mutation D75N slowed the kinetics of force development but did not affect the relaxation rate. The mutation P161S slowed both the relaxation and force development. The mutation D75N increased the calcium sensitivity of force, and the mutation P161S decreased it. The mutation D75N decreased the maximal isometric tension and increased the tension and stiffness at low calcium. Both mutations studied disrupt the calcium regulation of contractile force and affect the kinetics of its development and thus may impair cardiac diastolic function and cause myocardial hypertrophy.
Keywords: actin-myosin interaction; cardiac myosin binding protein C; fiber force kinetics; fiber stiffness; hypertrophic cardiomyopathy mutations; slow skeletal muscle fiber; tension recovery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Alfares A.A., Kelly M.A., McDermott G., Funke B.H., Lebo M.S., Baxter S.B., Shen J., McLaughlin H.M., Clark E.H., Babb L.J., et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genet. Med. 2015;17:880–888. doi: 10.1038/gim.2014.205. - DOI - PubMed
-
- Nagayama T., Takimoto E., Sadayappan S., Mudd J.O., Seidman J.G., Robbins J., Kass D.A. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: Protein kinase A phosphorylation dependent and independent regulation. Circulation. 2007;20:2399–2408. doi: 10.1161/CIRCULATIONAHA.107.706523. - DOI - PubMed
-
- Palmer B.M., Georgakopoulos D., Janssen P.M., Wang Y., Alpert N.R., Belardi D.F., Harris S.P., Moss R.L., Burgon P.G., Seidman C.E., et al. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ. Res. 2004;94:1249–1255. doi: 10.1161/01.RES.0000126898.95550.31. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

