Exploring the Osteoinductive Potential of Bacterial Pyomelanin Derived from Pseudomonas aeruginosa in a Human Osteoblast Model
- PMID: 39769171
- PMCID: PMC11678243
- DOI: 10.3390/ijms252413406
Exploring the Osteoinductive Potential of Bacterial Pyomelanin Derived from Pseudomonas aeruginosa in a Human Osteoblast Model
Abstract
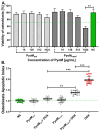
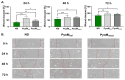
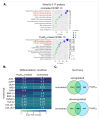
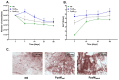
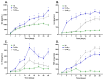
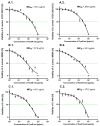
Alkaptonuria (AKU) is a genetically determined disease associated with disorders of tyrosine metabolism. In AKU, the deposition of homogentisic acid polymers contributes to the pathological ossification of cartilage tissue. The controlled use of biomimetics similar to deposits observed in cartilage during AKU potentially may serve the development of new bone regeneration therapy based on the activation of osteoblasts. The proposed biomimetic is pyomelanin (PyoM), a polymeric biomacromolecule synthesized by Pseudomonas aeruginosa. This work presents comprehensive data on the osteoinductive, pro-regenerative, and antibacterial properties, as well as the cytocompatibility, of water-soluble (PyoMsol) or water-insoluble (PyoMinsol) PyoM. Both variants of PyoM support osteoinductive processes as well as the maturation of osteoblasts in cell cultures in vitro due to the upregulation of bone-formation markers, osteocalcin (OC), and alkaline phosphatase (ALP). Furthermore, the cytokines involved in these processes were elevated in cell cultures of osteoblasts exposed to PyoM: tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10. The PyoM variants are cytocompatible in a wide concentration range and limit the doxorubicin-induced apoptosis of osteoblasts. This cytoprotective PyoM activity is correlated with an increased migration of osteoblasts. Moreover, PyoMsol and PyoMinsol exhibit antibacterial activity against staphylococci isolated from infected bones. The osteoinductive, pro-regenerative, and antiapoptotic effects achieved through PyoM stimulation prompt the development of new biocomposites modified with this bacterial biopolymer for medical use.
Keywords: bone regeneration; osteoblast; osteoinduction; pyomelanin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Can Pyomelanin Produced by Pseudomonas aeruginosa Promote the Regeneration of Gastric Epithelial Cells and Enhance Helicobacter pylori Phagocytosis?Int J Mol Sci. 2023 Sep 10;24(18):13911. doi: 10.3390/ijms241813911. Int J Mol Sci. 2023. PMID: 37762213 Free PMC article.
-
Bone marrow stromal cells generate an osteoinductive microenvironment when cultured on titanium-aluminum-vanadium substrates with biomimetic multiscale surface roughness.Biomed Mater. 2023 Mar 8;18(3):035001. doi: 10.1088/1748-605X/acbf15. Biomed Mater. 2023. PMID: 36827708 Free PMC article.
-
Betulin Promotes Differentiation of Human Osteoblasts In Vitro and Exerts an Osteoinductive Effect on the hFOB 1.19 Cell Line Through Activation of JNK, ERK1/2, and mTOR Kinases.Molecules. 2019 Jul 19;24(14):2637. doi: 10.3390/molecules24142637. Molecules. 2019. PMID: 31331121 Free PMC article.
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Progress and Applications of Polyphosphate in Bone and Cartilage Regeneration.Biomed Res Int. 2019 Jun 27;2019:5141204. doi: 10.1155/2019/5141204. eCollection 2019. Biomed Res Int. 2019. PMID: 31346519 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

