Identification, Phylogeny, and Expression Profiling of Pineapple Heat Shock Proteins (HSP70) Under Various Abiotic Stresses
- PMID: 39769172
- PMCID: PMC11678451
- DOI: 10.3390/ijms252413407
Identification, Phylogeny, and Expression Profiling of Pineapple Heat Shock Proteins (HSP70) Under Various Abiotic Stresses
Abstract
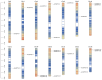
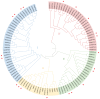
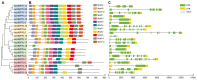
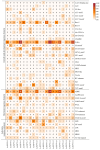
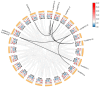
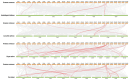
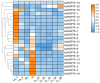
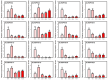
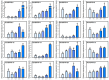
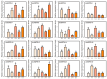
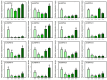
Pineapple (Ananas comosus (L.) Merr.) is an economically significant and delicious tropical fruit. Pineapple commercial production faces severe decline due to abiotic stresses, which affect the development and quality of pineapple fruit. Heat shock protein 70 (HSP70) plays an essential role in abiotic stress tolerance. However, the pineapple HSP70 family identification and expression analysis in response to abiotic stresses has not been studied. To explore the functional role of AcHSP70, different abiotic stress treatments were applied to pineapple cultivar "Bali" seedlings. A total of 21 AcHSP70 members were identified in the pineapple genome. The identified genes were classified into four subfamilies (I-IV) using phylogenetic analysis. The AcHSP70 family is expressed under different stress conditions. Quantitative real time polymerase chain reaction (qRT-PCR) revealed the expression pattern of the AcHSP70 family under cold, drought, salt, and heat stress. The expression level of genes such as AcHSP70-2 increased under heat, cold, and drought stress, while the expression level of genes such as AcHSP70-3 decreased under salt stress. Furthermore, the expression profile of AcHSP70s in different tissues and development stages was analyzed using transcriptome analysis. The HSP70 genes exhibited unique expression patterns in pineapple tissue at different developmental stages. The study therefore provides a list of HSP70 genes with substantial roles in abiotic stress response and valuable information for understanding AcHSP70 functional characteristics during abiotic stress tolerance in pineapple.
Keywords: AcHSP70 family; Ananas comosus; abiotic stress; gene expression; phylogeny.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Li D., Jing M., Dai X., Chen Z., Ma C., Chen J. Current Status of Pineapple Breeding, Industrial Development, and Genetics in China. Euphytica. 2022;218:85. doi: 10.1007/s10681-022-03030-y. - DOI
-
- Song K., Zhang X., Liu J., Yao Q., Li Y., Hou X., Liu S., Qiu X., Yang Y., Chen L., et al. Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes. Int. J. Mol. Sci. 2023;24:16384. doi: 10.3390/ijms242216384. - DOI - PMC - PubMed
-
- Lin W., Liu S., Xiao X., Sun W., Lu X., Gao Y., He J., Zhu Z., Wu Q., Zhang X. Integrative Analysis of Metabolome and Transcriptome Provides Insights into the Mechanism of Flower Induction in Pineapple (Ananas comosus (L.) Merr.) by Ethephon. Int. J. Mol. Sci. 2023;24:17133. doi: 10.3390/ijms242417133. - DOI - PMC - PubMed
-
- Sinaga A.O.Y., Marpaung D.S.S. Abiotic stress-induced gene expression in pineapple as a potential genetic marker. Adv. Agrochem. 2024;3:133–142. doi: 10.1016/j.aac.2024.01.005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

