Preliminary Study on the Positive Expression Regulation of Alpha2-Macroglobulin in the Testicular Tissue of Male Mice by Environmental Estrogens
- PMID: 39769199
- PMCID: PMC11676208
- DOI: 10.3390/ijms252413434
Preliminary Study on the Positive Expression Regulation of Alpha2-Macroglobulin in the Testicular Tissue of Male Mice by Environmental Estrogens
Abstract
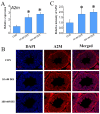
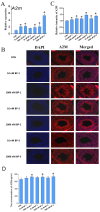
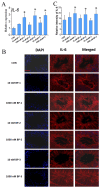
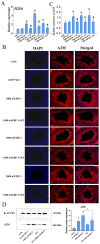
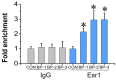
The male reproductive impairment caused by environmental estrogens (EEs) stands as a pivotal research area in environmental toxicology. Alpha2-macroglobulin (A2M) emerges as a promising molecule capable of counteracting oxidative stress induced by EEs. This study conducted exposure experiments spanning PND1 to PND56 employing ICR mice, aiming to delve into the expression patterns of A2M and its modulated IL-6 in the testicular tissue of mice subsequent to diethylstilbestrol (DES) and benzophenone (BP) exposure, while elucidating the pivotal role of ERs in this intricate process. Our findings revealed that upon DES exposure (10 and 100 nM), there was a pronounced upregulation of A2M (mRNA and in situ protein levels) in mouse testicular tissue. Similarly, exposure to BPs (BP-1, BP-2, and BP-3, each at 10 and 1000 nM) exhibited comparable effects and increasing A2M levels in serum. Notably, BP exposure also caused an elevation in IL-6 levels (which could be directly regulated by A2M) within testicular tissue (mRNA and in situ protein). Remarkably, the specific estrogen receptor antagonist ICI 182780 (0.5 mg/kg/day) was effective in reversing the upregulation of both A2M and IL-6 induced by BP exposure. Significantly, the results of theoretical prediction of the potential ERE site in the A2m gene promoter region and ChIP-qPCR experiment provide essential and strong evidence for the key conclusion that A2m is the target gene of ER. Taken together, our study highlights EEs' ability to regulate A2M expression in the male reproductive system via the ER signaling pathway. This vital insight deepens our understanding of molecular mechanisms protecting against oxidative stress caused by EEs.
Keywords: alpha2-macroglobulin (A2M); environmental estrogens (EEs); estrogen receptor (ER); interleukin-6 (IL-6); male reproductive system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Matta M.K., Zusterzeel R., Pilli N.R., Patel V., Volpe D.A., Florian J., Oh L., Bashaw E., Zineh I., Sanabria C., et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: A randomized clinical trial. JAMA. 2019;321:2082–2091. doi: 10.1001/jama.2019.5586. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- NYG2022030/Ningxia Higher Education Institution Scientific Research Project
- XT2023028/Ningxia High-level Talent Project
- 82274624/National Natural Science Foundation of China
- FP20230103/Ningxia Medical University Scientific Research Project
- FP20220204/Director Fund of the Key Laboratory of Fertility Preservation and Maintenance of Ministry of Education, Ningxia Medical University
LinkOut - more resources
Full Text Sources
Miscellaneous

