In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers
- PMID: 39769232
- PMCID: PMC11679655
- DOI: 10.3390/ijms252413469
In Vivo Induction of Leukemia-Specific Adaptive and Innate Immune Cells by Treatment of AML-Diseased Rats and Therapy-Refractory AML Patients with Blast Modulating Response Modifiers
Abstract
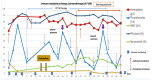
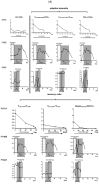
There is a high medical need to develop new strategies for the treatment of patients with acute myeloid leukemia (AML) refractory to conventional therapy. In vitro, the combinations of the blast-modulatory response modifiers GM-CSF + Prostaglandin E1, (summarized as Kit M) have been shown to convert myeloid leukemic blasts into antigen-presenting dendritic cells of leukemic origin (DCleu) that were able to (re-)activate the innate and adaptive immune system, direct it specifically against leukemic blasts, and induce memory cells. This study aimed to investigate the immune modulatory capacity and antileukemic efficacy of Kit M in vivo. Brown Norway rats suffering from AML were treated with Kit M (twofold application). Blasts and immune cells were monitored in peripheral blood (PB) and spleen. Upon the observation of promising immune modulatory effects in the treated animals, two patients with AML refractory to multiple lines of therapy were offered treatment with Kit M on an individualized basis. Safety, as well as immunological and clinical effects, were monitored. Samples obtained from a third patient in similar clinical conditions not receiving Kit M were used as controls for immune monitoring tests. Animal experiments: Drugs were well tolerated by the treated animals. After 9 days of treatment, DCleu and memory-like T cells increased in the peripheral blood, whereas regulatory T cells, especially blasts, decreased in treated as compared to untreated control animals. Clinical courses: No severe side effects were observed. In patient 1482, PB blasts remained under the detection threshold during 27 days of treatment, thrombocytes were normalized, and (leukemia specific) immune effector cells of the adaptive and innate immune system increased up to 800-fold compared to the start of treatment. Patient 1601 responded with a 12% reduction in blasts in PB immediately after Kit M treatment. Several subtypes of (leukemia-specific) immune effector cells in PB increased up to four-fold during the 19 days of treatment. In contrast, immune-reactive cells decreased under mild chemotherapy in the PB of control patient 1511 with comparably refractory AML. Within the limitation of low numbers in both animal experiments and clinical applications, our data suggest that Kit M treatment of AML-diseased rats and patients is feasible and may induce leukemia-specific immune reactions and clinical improvement. A larger series and a prospective clinical trial will be required to confirm our observations. Beyond optimized doses and schedules of the applied compounds, the combination with other antileukemic strategies or the application of Kit M in less proliferative stages of the myeloid diseases need to be discussed. If effects are confirmed, the concept may add to the armamentarium of treatments for highly aggressive blood cancer.
Keywords: AML; AML immunotherapy; immune-monitoring; kits; leukemia-derived dendritic cells; leukemia-specific cells; new AML drugs.
Conflict of interest statement
Modiblast Pharma GmbH (Oberhaching, Germany) holds the European Patent 15 801 987.7-1118 and US Patent 15-517627 ‘Use of immunomodulatory effective compositions for the immunotherapeutic treatment of patients suffering from myeloid leukemias’, with whom Schmetzer H. is involved with.
Figures


 pleural punctures.
pleural punctures.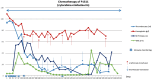

References
-
- Tamamyan G., Tervonen H., Mendoza L., Batmunkh T., Yap M.L., Rodin D., Moraes F.Y. Future of Global Cancer From the Perspective of Young Oncology Leaders. JGO. 2018;4:74s. doi: 10.1200/jgo.18.76600. - DOI
-
- O’Donnell M.R., Tallman M.S., Abboud C.N., Altman J.K., Appelbaum F.R., Arber D.A., Bhatt V., Bixby D., Blum W., Coutre S.E., et al. Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017;15:926–957. doi: 10.6004/jnccn.2017.0116. - DOI - PubMed
-
- Döhner H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H., Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. - DOI - PubMed
-
- Burnett A.K., Hills R.K., Nielsen O.J., Freeman S., Ali A., Cahalin P., Hunter A., Thomas I.F., Russell N.H. A comparison of FLAG-Ida and daunorubicin combined with clofarabine in high-risk acute myeloid leukaemia: Data from the UK NCRI AML17 Trial. Leukemia. 2018;32:2693–2697. doi: 10.1038/s41375-018-0148-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

