Interferon-α Inhibits NET Formation in Neutrophils Derived from Patients with Myeloproliferative Neoplasms in a Neutrophil Sub-Population-Specific Manner
- PMID: 39769242
- PMCID: PMC11677445
- DOI: 10.3390/ijms252413473
Interferon-α Inhibits NET Formation in Neutrophils Derived from Patients with Myeloproliferative Neoplasms in a Neutrophil Sub-Population-Specific Manner
Abstract
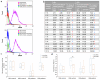
Neutrophils and neutrophil extracellular traps (NETs) contribute to thrombosis and hyperinflammation in myeloproliferative neoplasms (MPN). High-density neutrophils (HDNs) and low-density neutrophils (LDNs) have recently been characterized as distinct neutrophil sub-populations with distinct morphological and functional properties. We aim to study the kinetics of NET formation and inhibition with interferon-α (IFNα) in neutrophils derived from patients with MPN as compared to matched healthy controls. Ex vivo NET formation was assessed by neutrophil-elastase activity, neutrophil-associated nucleosomes, myeloperoxidase (MPO), and citrullinated histone H3 content. IFNα significantly inhibited NET formation in neutrophils derived from MPN patients. Neutrophil sub-population analysis demonstrated that HDNs drive the increase in NET formation as compared to LDNs in patients and in healthy controls and are effectively inhibited by IFNα, an effect that is lost in LDNs. In conclusion, we demonstrate that in MPN, HDNs drive excess NET formation and are more sensitive to IFNα inhibition. These observations uncover unique neutrophil sub-population biology and dynamics in MPN.
Keywords: high-density neutrophils; interferon-α; myeloproliferative neoplasia; neutrophil extracellular traps.
Conflict of interest statement
The authors declare no conflicts of interest. This article is a revised and expanded version of a paper which was presented at The 2024 American Society of Hematology annual meeting at San Diego, CA, USA [50].
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

