Immune Response Elicited by Recombinant Adenovirus-Delivered Glycoprotein B and Nucleocapsid Protein UL18 and UL25 of HSV-1 in Mice
- PMID: 39769249
- PMCID: PMC11678876
- DOI: 10.3390/ijms252413486
Immune Response Elicited by Recombinant Adenovirus-Delivered Glycoprotein B and Nucleocapsid Protein UL18 and UL25 of HSV-1 in Mice
Abstract
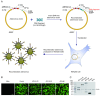
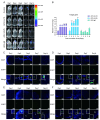
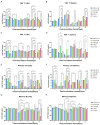
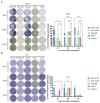
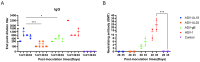
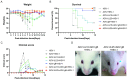
Due to the complex pathogenic and immune escape mechanisms of herpes simplex virus type 1 (HSV-1), especially the failure of induced immune responses to block the initial cell-to-cell transmission of the virus from skin cells to neurons, the body struggles to establish effective prevention and control methods, resulting in the failure of currently developed vaccines. Previous studies have highlighted the crucial roles of surface glycoproteins and nucleocapsid proteins in activating the body's immune defense system against HSV-1 infection. In this study, recombinant adenoviruses were used as vectors to generate adenoviruses carrying the nucleocapsid protein genes UL18 and UL25, as well as the surface glycoprotein gene gB. This approach aimed to mimic the protein expression process that occurs following viral infection of the host and to investigate the immune response characteristics induced by UL18, UL25, and gB proteins. The findings revealed that UL18, UL25, and gB proteins could all trigger the expression of genes associated with innate immune responses; however, the specific genes induced varied in type and level. Furthermore, all three proteins were capable of promoting the proliferation of CD8+ T cells in the lymph nodes. Notably, only UL18 and gB could elicit a Th1 cell immune response. Interestingly, among these proteins, only UL18 could also induce a relatively higher IL-4 level, indicating a Th2 cell immune response. In addition to cellular immunity, all three proteins stimulated the production of specific IgG antibodies. Notably, UL18 induced higher and more sustained levels of specific IgG antibodies in mice. By contrast, only glycoprotein gB induced lower levels of neutralizing antibodies in mice. Moreover, when these mice were challenged with HSV-1, the co-immunization with UL18 and gB provided better protection than gB alone. In conclusion, HSV-1 surface glycoproteins and nucleocapsid proteins exhibit differences in their ability to induce innate and adaptive immunity in the body, suggesting potential avenues for vaccine design by leveraging their complementary advantages.
Keywords: UL18; UL25; glycoprotein B; herpes simplex virus type 1; nucleocapsid protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- WHO. [(accessed on 5 April 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

