Itchy E3 Ubiquitin Ligase-Mediated Ubiquitination of Ferritin Light Chain Contributes to Endothelial Ferroptosis in Atherosclerosis
- PMID: 39769287
- PMCID: PMC11677933
- DOI: 10.3390/ijms252413524
Itchy E3 Ubiquitin Ligase-Mediated Ubiquitination of Ferritin Light Chain Contributes to Endothelial Ferroptosis in Atherosclerosis
Abstract
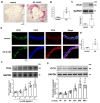
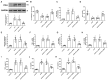
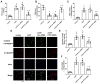
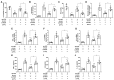
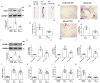
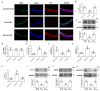
This research seeks to investigate the function and fundamental mechanisms of Itchy E3 ubiquitin ligase (ITCH), a HECT (homologous to E6AP carboxyl terminus)-type E3 ubiquitin ligase, in endothelial ferroptosis, particularly in the context of atherosclerosis, which has been underexplored. The levels of ITCH protein in the aortas of mice with atherosclerosis were analyzed. Constructs for ITCH RNA interference were generated and introduced into human aortic endothelial cells (HAECs). The findings indicated that ITCH protein expression was elevated in atherosclerotic mice and HAECs exposed to oxidized low-density lipoprotein (ox-LDL). ITCH downregulation significantly mitigated ox-LDL-induced endothelial injury and dysfunction. Reducing ITCH expression inhibited ox-LDL-induced endothelial ferroptosis. This study also revealed that ITCH mediates ox-LDL-induced ubiquitin-dependent degradation of ferritin light chain (FTL) in HAECs. The protective impact of ITCH knockdown against ox-LDL-induced ferroptosis and endothelial injury was reversed by FTL siRNA. Additionally, in vivo experiments showed that inhibiting ITCH reduced atherosclerosis progression and reversed ferroptosis in the aorta, with an associated increase in FTL protein expression in the aortas of mice. This study demonstrates that ITCH interacts with and regulates the stability of the FTL protein via the ubiquitin-proteasome system, contributing to ox-LDL-induced ferroptosis and endothelial cell dysfunction. Targeting components of the ITCH-FTL pathway holds potential as a therapeutic strategy against atherosclerosis.
Keywords: ITCH; atherosclerosis; endothelial cells; ferritin light chain; ferroptosis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

