A CsWRKY48 Gene from Tea Plants Intercropped with Chinese Chestnut Plays an Important Role in Resistance to Biotic and Abiotic Stresses
- PMID: 39769291
- PMCID: PMC11677473
- DOI: 10.3390/ijms252413526
A CsWRKY48 Gene from Tea Plants Intercropped with Chinese Chestnut Plays an Important Role in Resistance to Biotic and Abiotic Stresses
Abstract
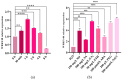
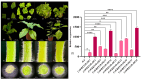
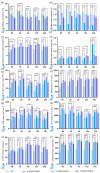
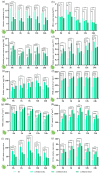
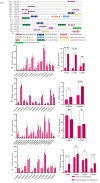
Tea plant (Camellia sinensis) is an important horticultural crop. The quality and productivity of tea plants is always threatened by various adverse environmental factors. Numerous studies have shown that intercropping tea plants with other plants can greatly improve the quality of their products. The intercropping system of Chinese chestnut (Castanea mollissima) and tea plants is an agricultural planting model in which the two species are grown on the same piece of land following a specific spacing and cultivation method. Based on a comparative transcriptome analysis between Chinese chestnut tea intercropped plantations and a pure tea plantation, it was found that the expression levels of the WRKY genes were significantly upregulated under the intercropping pattern. In this study, we cloned a candidate gene, CsWRKY48, and verified its functions in tobacco (Nicotiana tabacum) via heterologous transformation. The contents of protective enzyme activities and osmoregulatory substances were significantly increased, and the trichomes length and density were improved in the transgenic tobacco lines. This phenotype offered an enhanced resistance to both low temperatures and aphids for transgenic lines overexpressing CsWRKY48. Further analysis indicated that the CsWRKY48 transcription factor might interact with other regulators, such as CBF, ERF, MYC, and MYB, to enhance the resistance of tea plants to biotic and abiotic stresses. These findings not only confirm the elevated resistance of tea plants under intercropping, but also indicate a potential regulatory network mediated by the WRKY transcription factor.
Keywords: CsWRKY48; cold resistance; insect resistance; tea plants intercropped with Chinese chestnut; trichomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
CsAFS1 Enhances the Self-Resistance of Tea Plants by Promoting Terpenoid Accumulation.Physiol Plant. 2025 Jul-Aug;177(4):e70430. doi: 10.1111/ppl.70430. Physiol Plant. 2025. PMID: 40730416
-
Genome-wide identification and expression pattern analysis of WRKY transcription factors in response to biotic and abiotic stresses in tea plants (Camellia sinensis).Plant Physiol Biochem. 2024 Jun;211:108670. doi: 10.1016/j.plaphy.2024.108670. Epub 2024 Apr 28. Plant Physiol Biochem. 2024. PMID: 38703501
-
Non-targeted and targeted metabolomics profiling of tea plants (Camellia sinensis) in response to its intercropping with Chinese chestnut.BMC Plant Biol. 2021 Jan 21;21(1):55. doi: 10.1186/s12870-021-02841-w. BMC Plant Biol. 2021. PMID: 33478393 Free PMC article.
-
Biosynthesis and the Transcriptional Regulation of Terpenoids in Tea Plants (Camellia sinensis).Int J Mol Sci. 2023 Apr 8;24(8):6937. doi: 10.3390/ijms24086937. Int J Mol Sci. 2023. PMID: 37108101 Free PMC article. Review.
-
Advances in research on functional genes of tea plant.Gene. 2019 Aug 30;711:143940. doi: 10.1016/j.gene.2019.143940. Epub 2019 Jun 18. Gene. 2019. PMID: 31226279 Review.
Cited by
-
Glutathione S-transferase CrGST24 in the differentiation of adventitious buds from Camellia reticulata callus.Front Plant Sci. 2025 Aug 15;16:1641401. doi: 10.3389/fpls.2025.1641401. eCollection 2025. Front Plant Sci. 2025. PMID: 40894507 Free PMC article.
References
-
- Wang Y., He W., Wang L., Lan Y.X., Wu M. TCP transcription factor identification in pecan (Carya illinoensis) and salt tolerance function analysis of CiTCP8. Sci. Hortic. 2024;13:2568. doi: 10.1016/j.scienta.2024.113051. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

