The Motility of Mouse Spermatozoa Changes Differentially After 30-Minute Exposure Under Simulating Weightlessness and Hypergravity
- PMID: 39769324
- PMCID: PMC11678010
- DOI: 10.3390/ijms252413561
The Motility of Mouse Spermatozoa Changes Differentially After 30-Minute Exposure Under Simulating Weightlessness and Hypergravity
Abstract
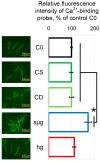
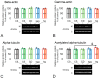
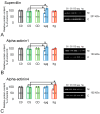
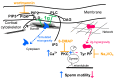
Research into the mechanisms by which gravity influences spermatozoa has implications for maintaining the species in deep space exploration and may provide new approaches to reproductive technologies on Earth. Changes in the speed of mouse spermatozoa after 30 min exposure to simulated weightlessness (by 3D-clinostat) and 2 g hypergravity (by centrifugation) were studied using inhibitory analysis. Simulated microgravity after 30 min led to an increase in the speed of spermatozoa and against the background of an increase in the relative calcium content in the cytoplasm. This effect was prevented by the introduction of 6-(dimethylamino) purine, wortmannin, and calyculin A. Hypergravity led to a decrease in the speed of spermatozoa movement, which was prevented by sodium orthovanadate and calyculin A. At the same time, under microgravity conditions, there was a redistribution of proteins forming microfilament bundles between the membrane and cytoplasmic compartments and under hypergravity conditions-proteins forming networks. The obtained results indicate that even a short exposure of spermatozoa to altered gravity leads to the launch of mechanotransduction pathways in them and a change in motility.
Keywords: cell mechanosensitivity; cytoskeleton; hypergravity; microgravity; spermatozoa.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Serova L.V., Denisova L.A., Apanasenko Z.I., Kuznetsova M.A., Meizerov E.S. Reproductive function of the male rat after a flight on the Kosmos-1129 biosatellite. Kosm. Biol. Aviakosm. Med. 1982;16:62–65. - PubMed
-
- Serova L.V. Effect of weightlessness on the reproductive system of mammals. Kosm. Biol. Aviakosm. Med. 1989;23:11–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

