Electrochemical Degradation of Sulfamethoxazole Enhanced by Bio-Inspired Iron-Nickel Encapsulated Biochar Particle Electrode
- PMID: 39769341
- PMCID: PMC11678343
- DOI: 10.3390/ijms252413579
Electrochemical Degradation of Sulfamethoxazole Enhanced by Bio-Inspired Iron-Nickel Encapsulated Biochar Particle Electrode
Abstract
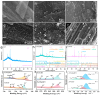
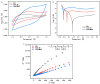
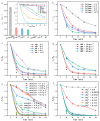
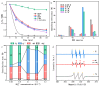
In the electrocatalytic (EC) degradation process, challenges such as inefficient mass transfer, suboptimal mineralization rates, and limited current efficiency have restricted its broader application. To overcome these obstacles, this study synthesized spherical particle electrodes (FeNi@BC) with superior electrocatalytic performance using a bio-inspired preparation method. A three-dimensional electrocatalytic oxidation system based on FeNi@BC electrode, EC/FeNi@BC, showed excellent degradation efficiency of sulfamethoxazole (SMX), reaching 0.0456 min-1. Quenching experiments and electron paramagnetic resonance experiments showed that the excellent SMX degradation efficiency in the EC/FeNi@BC system was attributed to the synergistic effect of multiple reactive oxygen species (ROS) and revealed their evolution path. Characterization results showed that FeNi3 generated in the FeNi@BC electrode was a key bimetallic active site for improving electrocatalytic activity and repolarization ability. More importantly, the degradation pathway and reaction mechanism of SMX in the EC/FeNi@BC system were proposed. In addition, the influencing factors of the reaction system (voltage, pH, initial SMX concentration, electrode dosage, and sodium sulfate concentration, etc.) and the stability of the catalyst (maintained more than 81% after 5 cycles) were systematically evaluated. This study may provide help for the construction of environmentally friendly catalytic and efficient degradation of organic pollutants.
Keywords: FeNi@BC; bio-inspired catalyst; electrocatalysis; particle electrode; sulfamethoxazole.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Chen F., Liu L.-L., Chen J.-J., Li W.-W., Chen Y.-P., Zhang Y.-J., Wu J.-H., Mei S.-C., Yang Q., Yu H.-Q. Efficient decontamination of organic pollutants under high salinity conditions by a nonradical peroxymonosulfate activation system. Water Res. 2021;191:116799. doi: 10.1016/j.watres.2020.116799. - DOI - PubMed
-
- Guo S.-T., Tang Z.-Y., Du Y.-W., Liu T., Ouyang T., Liu Z.-Q. Chlorine anion stabilized Cu2O/ZnO photocathode for selective CO2 reduction to CH4. Appl. Catal. B Environ. 2023;321:122035. doi: 10.1016/j.apcatb.2022.122035. - DOI
-
- Yao F., Jia M., Yang Q., Chen F., Zhong Y., Chen S., He L., Pi Z., Hou K., Wang D., et al. Highly selective electrochemical nitrate reduction using copper phosphide self-supported copper foam electrode: Performance, mechanism, and application. Water Res. 2021;193:116881. doi: 10.1016/j.watres.2021.116881. - DOI - PubMed
-
- Bu J., Deng Z., Liu H., Li T., Yang Y., Zhong S. The degradation of sulfamilamide wastewater by three-dimensional electrocatalytic oxidation system composed of activated carbon bimetallic particle electrode. J. Clean. Prod. 2021;324:129256. doi: 10.1016/j.jclepro.2021.129256. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

