Characterization of Dendritic Cells and Myeloid-Derived Suppressor Cells Expressing Major Histocompatibility Complex Class II in Secondary Lymphoid Organs in Systemic Lupus Erythematosus-Prone Mice
- PMID: 39769367
- PMCID: PMC11676837
- DOI: 10.3390/ijms252413604
Characterization of Dendritic Cells and Myeloid-Derived Suppressor Cells Expressing Major Histocompatibility Complex Class II in Secondary Lymphoid Organs in Systemic Lupus Erythematosus-Prone Mice
Abstract
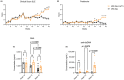
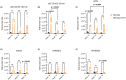
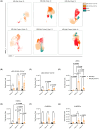
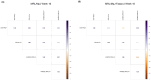
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by self-antibody production and widespread inflammation affecting various body tissues. This disease is driven by the breakdown of immune tolerance, which promotes the activation of autoreactive B and T cells. A key feature of SLE is dysregulation in antigen presentation, where antigen-presenting cells (APCs) play a central role in perpetuating immune responses. Dendritic cells (DCs) are highly specialized for antigen presentation among APCs. At the same time, myeloid-derived suppressor cells (MDSCs) can also express MHC-II molecules, although their role in SLE is less understood. Utilizing the SLE model, MRL/MpJ-Faslpr/J, we determined the presence of different phenotypes of DCs and MDSCs expressing MHC-II in secondary lymphoid organs, along with the gene expression of ICOSL, CD80 and CD86 in the spleen. Our study determined that the most abundant population of APCs in secondary lymphoid organs corresponds to cDC CD103-CD11b+ MHC-II+ throughout SLE development. Additionally, ICOSL expression increased over time, becoming more preponderant in week 16 in the SLE model, which could indicate that it is a crucial pathway for the development and progression of the pathology. In week 16, we observed a positive correlation between M-MDSC MHC-II and IFN-γ-producing CD4+ T cells.
Keywords: MRL/MpJ-Faslpr/J; co-stimulatory molecules; dendritic cells (DCs); innate immunity; major histocompatibility complex class II (MHC-II); myeloid-derived suppressor cells (MDSCs); systemic lupus erythematosus (SLE).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Waithman J., Moffat J.M., Patterson N.L., van Beek A.E., Mintern J.D. Reference Module in Biomedical Sciences. Elsevier; Amsterdam, The Netherlands: 2014. Antigen Presentation.
MeSH terms
Substances
Grants and funding
- 1231866/Agencia Nacional de Investigación y Desarrollo (ANID)
- 11221280/Agencia Nacional de Investigación y Desarrollo (ANID)
- SA77210051/Programa de Atracción e Inserción de capital humano avanzado (PAI)
- CN09_016 / ICN 2021_045; former P09/016-F/Millennium Institute on Immunology and Immunotherapy
- 21240988/Beca Doctorado Nacional ANID
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

