Divergent Contribution of Cytoplasmic Actins to Nuclear Structure of Lung Cancer Cells
- PMID: 39769373
- PMCID: PMC11727787
- DOI: 10.3390/ijms252413607
Divergent Contribution of Cytoplasmic Actins to Nuclear Structure of Lung Cancer Cells
Abstract
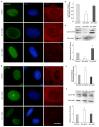
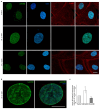
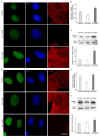
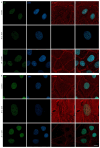
A growing body of evidence suggests that actin plays a role in nuclear architecture, genome organisation, and regulation. Our study of human lung adenocarcinoma cells demonstrates that the equilibrium between actin isoforms affects the composition of the nuclear lamina, which in turn influences nuclear stiffness and cellular behaviour. The downregulation of β-actin resulted in an increase in nuclear area, accompanied by a decrease in A-type lamins and an enhancement in lamin B2. In contrast, the suppression of γ-actin led to upregulation of the lamin A/B ratio through an increase in A-type lamins. Histone H3 post-translational modifications display distinct patterns in response to decreased actin isoform expression. The level of dimethylated H3K9me2 declined while acetylated H3K9ac increased in β-actin-depleted A549 cells. In contrast, the inhibition of γ-actin expression resulted in a reduction in H3K9ac. Based on our observations, we propose that β-actin plays a role in chromatin compaction and deactivation, and is involved in the elevation of nuclear stiffness through the control of the lamins ratio. The non-muscle γ-actin is presumably responsible for chromatin decondensation and activation. The identification of novel functions for actin isoforms offers insights into the mechanisms through which they influence cell fate during development and cancer progression.
Keywords: chromatin; histone; lamin; nucleus; β-actin; γ-actin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
β- and γ-Actins in the nucleus of human melanoma A375 cells.Histochem Cell Biol. 2015 Nov;144(5):417-28. doi: 10.1007/s00418-015-1349-8. Epub 2015 Aug 4. Histochem Cell Biol. 2015. PMID: 26239425 Free PMC article.
-
Nuclear envelope remodelling during human spermiogenesis involves somatic B-type lamins and a spermatid-specific B3 lamin isoform.Mol Hum Reprod. 2015 Mar;21(3):225-36. doi: 10.1093/molehr/gau111. Epub 2014 Dec 4. Mol Hum Reprod. 2015. PMID: 25477337
-
Increased Lamin B1 Levels Promote Cell Migration by Altering Perinuclear Actin Organization.Cells. 2020 Sep 24;9(10):2161. doi: 10.3390/cells9102161. Cells. 2020. PMID: 32987785 Free PMC article.
-
Lamins: The backbone of the nucleocytoskeleton interface.Curr Opin Cell Biol. 2024 Feb;86:102313. doi: 10.1016/j.ceb.2023.102313. Epub 2024 Jan 22. Curr Opin Cell Biol. 2024. PMID: 38262116 Review.
-
Laminopathies: what can humans learn from fruit flies.Cell Mol Biol Lett. 2018 Jul 6;23:32. doi: 10.1186/s11658-018-0093-1. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30002683 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

