Expression Levels of MUC5AC and MUC5B in Airway Goblet Cells Are Associated with Traits of COPD and Progression of Chronic Airflow Limitation
- PMID: 39769414
- PMCID: PMC11678853
- DOI: 10.3390/ijms252413653
Expression Levels of MUC5AC and MUC5B in Airway Goblet Cells Are Associated with Traits of COPD and Progression of Chronic Airflow Limitation
Abstract
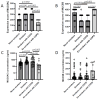
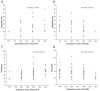
Mucins 5AC (MUC5AC) and 5B (MUC5B) are the major mucins providing the organizing framework for the airway's mucus gel. We retrieved bronchial mucosal biopsies and bronchial wash (BW) samples through bronchoscopy from patients with chronic obstructive pulmonary disease (n = 38), healthy never-smokers (n = 40), and smokers with normal lung function (n = 40). The expression of MUC5AC and MUC5B was assessed immunohistochemically. The mucin concentrations in BW were determined using the slot-blot technique. The immunohistochemical expression of MUC5AC and MUC5B was localized to goblet cells and submucosal glands. Smokers had higher MUC5AC and lower MUC5B goblet cell expression and higher concentrations of soluble MUC5AC in BW than never-smokers. The MUC5B expression in goblet cells correlated positively with expiratory air flows, diffusing capacity, and the dyspnoea score. Chronic bronchitis, emphysema, and the progression of chronic airflow limitation during a median follow-up time of 8.4 years were associated with higher MUC5AC and lower MUC5B expression in goblet cells. Sustainers, slow progressors, and rapid progressors of airflow obstruction differed in their MUC5B expression at baseline. Emphysema and bronchial wall thickening on CT at a follow-up visit were associated with lower MUC5B expression at baseline. Our findings strengthen the hypothesis that MUC5AC and MUC5B are yet another contributing factor to smoking-associated lung disease progression.
Keywords: COPD; epithelium; immunohistochemistry; mucin; smoking.
Conflict of interest statement
All authors declare they have no conflicts of interest.
Figures




References
-
- Hogg J.C., Chu F.S.F., Tan W.C., Sin D.D., Patel S.A., Pare P.D., Martinez F.J., Rogers R.M., Make B.J., Criner G.J., et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: Insights from small airway pathology: Insights from small airway pathology. Am. J. Respir. Crit. Care Med. 2007;176:454–459. doi: 10.1164/rccm.200612-1772OC. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- NA/The Foundation of the Finnish Anti-Tuberculosis Association
- NA/The Jalmari and Rauha Ahokas Foundation
- NA/The Research Foundation of the Pulmonary Diseases
- NA/A state subsidy of Oulu University Hospital
- CMS 20100435, 20130293, 20160418, 20180235, 20190320; SL 20230626/The Swedish Heart-Lung Foundation
- CMS 2016/The Swedish Respiratory Society
- CMS 2011, 2012, 2013/King Gustaf V's and Queen Victoria's Freemasons' Foundation
- CMS 2010/Hesselmans Foundation
- NA/Karolinska Institutet
- 2017-01142, 2018-00520 and 2021-02542/The Swedish Research Council
- CMS 071217/The Swedish Cancer- and Allergy Foundation
- CMS 2013/Sandoz A/S
- CMS 20110434, 20130051, 20150061, 20170393 and 20200287/The Regional Agreement on Medical Training and Clinical Research (ALF) between Stockholm County Council and Karolinska Institutet
- MCF [7214]-2013/ERS-EU RESPIRE2
LinkOut - more resources
Full Text Sources
Medical

