Alkaloids from Waltheria spp. (Malvaceae): Chemosystematic Aspects, Biosynthesis, Total Synthesis, and Biological Activities
- PMID: 39769421
- PMCID: PMC11727749
- DOI: 10.3390/ijms252413659
Alkaloids from Waltheria spp. (Malvaceae): Chemosystematic Aspects, Biosynthesis, Total Synthesis, and Biological Activities
Abstract
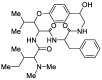
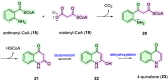
Waltheria, a genus within the Malvaceae family, is abundantly distributed in tropical and subtropical areas worldwide. Many species of this genus are widely utilized in various ways, including chewing, in folk medicine, acting as an anti-inflammatory agent, and treating gastrointestinal disorders, rheumatism, and asthma, among other conditions. These applications are largely due to their secondary metabolites, primarily quinolone alkaloids and cyclopeptides. Several biological activities have been reported for Waltheria species, including antifungal, anticancer, trypanocidal, acetylcholinesterase inhibitory, potential anti-HIV, antinociceptive, analgesic, anti-inflammatory, antibacterial, antioxidant, and leishmanicidal activities. This review not only presents information on isolated alkaloids and their biological activities but also delves into biosynthetic, chemosystematic, medicinal chemistry, and total synthesis aspects. Additionally, the manuscript highlights other applications of alkaloids of the genus, such as a study on their herbicidal activity, which shows significant potential for agricultural use.
Keywords: Waltheria; alkaloids; antidesmone; natural products; waltheriones.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

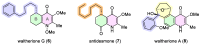
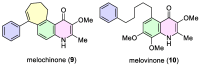









Similar articles
-
Dereplication of 4-Quinolone Alkaloids from Waltheria Indica (Malvaceae) Tissues Using Molecular Network Tools.Chem Biodivers. 2024 Aug;21(8):e202400665. doi: 10.1002/cbdv.202400665. Epub 2024 Jul 3. Chem Biodivers. 2024. PMID: 38825721
-
Identification of plant metabolite classes from Waltheria Indica L. extracts regulating inflammatory immune responses via COX-2 inhibition.J Ethnopharmacol. 2021 Apr 24;270:113741. doi: 10.1016/j.jep.2020.113741. Epub 2021 Jan 13. J Ethnopharmacol. 2021. PMID: 33359867
-
Aspidosperma species: A review of their chemistry and biological activities.J Ethnopharmacol. 2019 Mar 1;231:125-140. doi: 10.1016/j.jep.2018.10.039. Epub 2018 Nov 3. J Ethnopharmacol. 2019. PMID: 30395977 Review.
-
Anti-inflammatory activities of Waltheria indica extracts by modulating expression of IL-1B, TNF-α, TNFRII and NF-κB in human macrophages.Inflammopharmacology. 2020 Apr;28(2):525-540. doi: 10.1007/s10787-019-00658-6. Epub 2019 Nov 4. Inflammopharmacology. 2020. PMID: 31686273
-
A review of the safety profile, antioxidant, anti-inflammatory, and bronchorelaxant activities of Waltheria indica Linn (Malvaceae): A potential antiasthmatic phytomedicine.Heliyon. 2024 Jun 6;10(12):e32402. doi: 10.1016/j.heliyon.2024.e32402. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38975151 Free PMC article. Review.
Cited by
-
Next-generation antifungal drugs: Mechanisms, efficacy, and clinical prospects.Acta Pharm Sin B. 2025 Aug;15(8):3852-3887. doi: 10.1016/j.apsb.2025.06.013. Epub 2025 Jun 23. Acta Pharm Sin B. 2025. PMID: 40893690 Free PMC article. Review.
References
-
- WFO The World Flora Online. [(accessed on 10 April 2023)]. Available online: http://www.worldfloraonline.org/taxon/wfo-4000040514.
-
- Coutinho T.S., Colli-Silva M., Alves M. Novelties in Brazilian Waltheria L. (Byttnerioideae, Malvaceae): Two New Species and One Re-Establishment. Acta Bot. Bras. 2020;34:449–459. doi: 10.1590/0102-33062020abb0088. - DOI
-
- Coutinho T.S., Sader M.A., Pedrosa-harand A., Alves M. Waltheria marielleae (Byttnerioideae, Malvaceae), a New Species from North-Eastern Brazil Supported by Morphological and Phylogenetic Evidence. Plant Ecol. Evol. 2022;155:353–362. doi: 10.5091/plecevo.94921. - DOI
-
- Coutinho T.S., Colli-Silva M., Pirani J.R. Flora do Brasil 2020. Jardim Botânico do Rio de Janeiro; Rio de Janeiro, Brazil: 2024. Waltheria L.
-
- Viegas C.C.d.S.D., Rodrigues L.T.D., Pires H.F.O., de Assis T.S. Propriedades Bioativas in vitro e in vivo do Gênero Waltheria Pertencente à Família Malvaceae: Uma Revisão da Literatura. Res. Soc. Dev. 2022;11:e19011427351. doi: 10.33448/rsd-v11i4.27351. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

