Lactobacillus agilis SNF7 Presents Excellent Antibacteria and Anti-Inflammation Properties in Mouse Diarrhea Induced by Escherichia coli
- PMID: 39769422
- PMCID: PMC11728428
- DOI: 10.3390/ijms252413660
Lactobacillus agilis SNF7 Presents Excellent Antibacteria and Anti-Inflammation Properties in Mouse Diarrhea Induced by Escherichia coli
Abstract
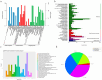
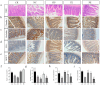
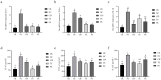
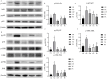
Escherichia coli (E. coli) is a common pathogen that causes diarrhea in newborns and animals. Antibiotics are typically used to treat bacterial diarrhea, a global intestinal health issue. Probiotics have gained interest as a potential substitute for antibiotics in the management of E. coli-induced diarrhea and present novel therapeutic options. In this study, the probiotic properties of Lactobacillus agilis SNF7 (L. agilis SNF7) isolated from feces were investigated, and whole genome sequencing was performed to evaluate the properties of the strain. Furthermore, we investigated the protective effects of L. agilis SNF7 in a mouse model of E. coli K99 infection. L. agilis SNF7 exhibits a high survival rate in artificial gastroenteric fluid and bile salt environments, along with an antagonistic effect against E. coli O111:K58 (B4), Staphylococcus aureus (S. aureus), and E. coli K99. Multiple genes with probiotic properties, including bacteriostasis, anti-inflammation, antioxidant, CAZyme, and the utilization of carbohydrate compounds, were identified in genome. L. agilis SNF7 prevented the gut barrier from being damaged by E. coli K99, reducing the clinical manifestations of the infection. Furthermore, L. agilis SNF7 reduced the expression of inflammatory cytokines (IL-6, IL-1β, and TNF-α) by inhibiting the phosphorylation of proteins linked to the NF-κB and MAPK signaling pathways. L. agilis SNF7 improved the intestinal microbial barrier, controlled the balance of the intestinal microecology, and reduced the entry of harmful microbes into the intestine. By controlling gut flora and reducing the inflammatory response, L. agilis SNF7 may be able to prevent and treat E. coli K99 infections. The application of L. agilis SNF7 in the creation of probiotic formulations to stop intestinal illnesses brought on by E. coli infections is clarified by this work.
Keywords: Escherichia coli; Lactobacillus agilis SNF7; diarrhea; probiotic properties; whole genome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- Ni X. Application progress of autochthonous microbiota in broiler feed. Feed Ind. 2021;42:1–4. doi: 10.13302/j.cnki.fi.2021.05.001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

