Suppression of Pathological Allergen-Specific B Cells by Protein-Engineered Molecules in a Mouse Model of Chronic House Dust Mite Allergy
- PMID: 39769423
- PMCID: PMC11728213
- DOI: 10.3390/ijms252413661
Suppression of Pathological Allergen-Specific B Cells by Protein-Engineered Molecules in a Mouse Model of Chronic House Dust Mite Allergy
Abstract
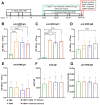
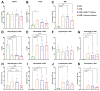
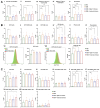
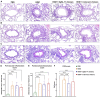
Der p1 is one of the major allergens causing house dust mite (HDM) allergy. Pathological Der p1-specific B cells play a key role in allergic inflammation as producers of allergen-specific antibodies. Crosslinking the inhibitory FcγRIIb with the B cell receptor triggers a high-affinity suppressive signal in B cells. Selective elimination of allergen-specific cells could potentially be achieved by administering chimeric molecules that combine, through protein engineering, the FcγRIIb-targeting monoclonal 2.4G2 antibody with the epitope-carrying Dp52-71 peptides from Der p1. We tested this hypothesis, in a chronic mouse model of HDM allergy induced in BalB/c mice, using FACS and ELISA assays, along with histopathological and correlational analyses. Dp52-71chimera treatment of HDM-challenged mice led to a decrease in serum anti-HDM IgG1 antibodies, a reduction in BALF β-hexosaminidase levels, a lowered number of SiglecFhigh CD11clow eosinophils, and an improved lung PAS score. Furthermore, we observed overexpression of FcγRIIb on the surface of CD19 cells in the lungs of HDM-challenged animals, which negatively correlated with the levels of lung alveolar macrophages, neutrophils, and BALF IL-13. Taken together, these results suggest that the use of FcγRIIb overexpression, combined with the expansion of chimeric protein technology to include more epitopes, could improve the outcome of inflammation.
Keywords: FcγRIIb receptors; chimeric molecules; chronic house dust mite allergy model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sánchez-Borges M., Fernandez-Caldas E., Thomas W.R., Chapman M.D., Lee B.W., Caraballo L., Acevedo N., Chew F.T., Ansotegui I.J., Behrooz L., et al. International consensus (ICON) on: Clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ. J. 2017;10:14. doi: 10.1186/s40413-017-0145-4. - DOI - PMC - PubMed
-
- Malone M. Review: Side Effects of Some Commonly Used Allergy Medications (Decongestants, Anti-Leukotriene Agents, Antihistamines, Steroids, and Zinc) and Their Safety in Pregnancy. Int. J. Allergy Medicat. 2017;3:24. doi: 10.23937/2572-3308.1510024. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

