LPS Disrupts Endometrial Receptivity by Inhibiting STAT1 Phosphorylation in Sheep
- PMID: 39769435
- PMCID: PMC11678167
- DOI: 10.3390/ijms252413673
LPS Disrupts Endometrial Receptivity by Inhibiting STAT1 Phosphorylation in Sheep
Abstract
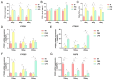
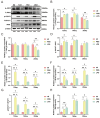
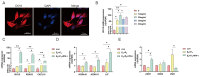
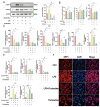
Uterine infections reduce ruminant reproductive efficiency. Reproductive dysfunction caused by infusion of Gram-negative bacteria is characterized by the failure of embryo implantation and reduced conception rates. Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria, is highly abortogenic. In this study, the effects of LPS infusion on the endometrial receptivity of sheep were studied during three critical periods of embryo implantation. The results showed that LPS infusion on d12, d16, and d20 of pregnancy in vivo interfered with the expression of prostaglandins (PGs) and affected the expression of adhesion-related factors (ITGB1/3/5, SPP1), key implantation genes (HOXA10, HOXA11 and LIF), and progestational elongation genes (ISG15, RSAD2 and CXCL10) during embryo implantation. In addition, after LPS infusion on d12, d16, and d20, the phosphorylation level of STAT1 significantly decreased and the protein expression level of IRF9 significantly increased on d12, suggesting that LPS infusion in sheep impairs endometrial receptivity through the JAK2/STAT1 pathway. Sheep endometrial epithelial cells were treated with 17 β-estrogen, progesterone, and/or interferon-tau in vitro to mimic the receptivity of the endometrium during early pregnancy for validation. LPS and the p-STAT1 inhibitor fludarabine were both added to the model, which resulted in reduced p-STAT1 protein expression, significant inhibition of PGE2/PGF2α, and significant suppression of the expression of key embryo implantation genes. Collectively, these results indicate that LPS infusion in sheep on d12, d16, and d20 impairs endometrial receptivity through the JAK2/STAT1 pathway, which is responsible for LPS-associated pregnancy failure.
Keywords: LPS; STAT1; embryo implantation; endometrial receptivity; sheep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yang Q., Liu J., Wang Y., Zhao W., Wang W., Cui J., Yang J., Yue Y., Zhang S., Chu M., et al. A proteomic atlas of ligand–receptor interactions at the ovine maternal–fetal interface reveals the role of histone lactylation in uterine remodeling. J. Biol. Chem. 2021;298:101456. doi: 10.1016/j.jbc.2021.101456. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

