Current Non-Viral-Based Strategies to Manufacture CAR-T Cells
- PMID: 39769449
- PMCID: PMC11728233
- DOI: 10.3390/ijms252413685
Current Non-Viral-Based Strategies to Manufacture CAR-T Cells
Abstract
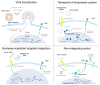
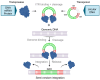
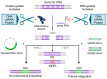
The successful application of CAR-T cells in the treatment of hematologic malignancies has fundamentally changed cancer therapy. With increasing numbers of registered CAR-T cell clinical trials, efforts are being made to streamline and reduce the costs of CAR-T cell manufacturing while improving their safety. To date, all approved CAR-T cell products have relied on viral-based gene delivery and genomic integration methods. While viral vectors offer high transfection efficiencies, concerns regarding potential malignant transformation coupled with costly and time-consuming vector manufacturing are constant drivers in the search for cheaper, easier-to-use, safer, and more efficient alternatives. In this review, we examine different non-viral gene transfer methods as alternatives for CAR-T cell production, their advantages and disadvantages, and examples of their applications. Transposon-based gene transfer methods lead to stable but non-targeted gene integration, are easy to handle, and achieve high gene transfer rates. Programmable endonucleases allow targeted integration, reducing the potential risk of integration-mediated malignant transformation of CAR-T cells. Non-integrating CAR-encoding vectors avoid this risk completely and achieve only transient CAR expression. With these promising alternative techniques for gene transfer, all avenues are open to fully exploiting the potential of next-generation CAR-T cell therapy and applying it in a wide range of applications.
Keywords: CAR-T cells; CRISPR; gene transfer; non-integrating; non-viral; piggybac; programmable endonuclease; sleeping beauty; transient; transposase.
Conflict of interest statement
MH and SRF are listed as inventors of patent applications and granted patents related to CAR-T technologies that have been filed by the Fred Hutchinson Cancer Research Center, Seattle, WA, and by the University of Würzburg, Würzburg, Germany. MH is the co-founder and equity owner of T-CURX GmbH, Würzburg, Germany. VG and SRF hold secondary employment at T-CURX GmbH. MH received honoraria from Celgene/BMS, Janssen, and Kite/Gilead. HE received honoraria from Pfizer, Amgen, Janssen, Sanofi, and BMS. The remaining authors declare that their research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Prommersberger S., Reiser M., Beckmann J., Danhof S., Amberger M., Quade-Lyssy P., Einsele H., Hudecek M., Bonig H., Ivics Z. CARAMBA: A first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. 2021;28:560–571. doi: 10.1038/s41434-021-00254-w. - DOI - PMC - PubMed
-
- Gogol-Döring A., Ammar I., Gupta S., Bunse M., Miskey C., Chen W., Uckert W., Schulz T.F., Izsvák Z., Ivics Z. Genome-wide Profiling Reveals Remarkable Parallels Between Insertion Site Selection Properties of the MLV Retrovirus and the piggyBac Transposon in Primary Human CD4+ T Cells. Mol. Ther. 2016;24:592–606. doi: 10.1038/mt.2016.11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

