Inhibiting the Cholesterol Storage Enzyme ACAT1/SOAT1 in Aging Apolipoprotein E4 Mice Alters Their Brains' Inflammatory Profiles
- PMID: 39769453
- PMCID: PMC11727783
- DOI: 10.3390/ijms252413690
Inhibiting the Cholesterol Storage Enzyme ACAT1/SOAT1 in Aging Apolipoprotein E4 Mice Alters Their Brains' Inflammatory Profiles
Abstract
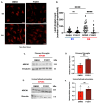
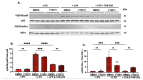
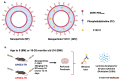
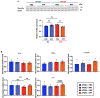
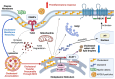
Aging and apolipoprotein E4 (APOE4) are the two most significant risk factors for late-onset Alzheimer's disease (LOAD). Compared to APOE3, APOE4 disrupts cholesterol homeostasis, increases cholesteryl esters (CEs), and exacerbates neuroinflammation in brain cells, including microglia. Targeting CEs and neuroinflammation could be a novel strategy to ameliorate APOE4-dependent phenotypes. Toll-like receptor 4 (TLR4) is a key macromolecule in inflammation, and its regulation is associated with the cholesterol content of lipid rafts in cell membranes. We previously demonstrated that in normal microglia expressing APOE3, inhibiting the cholesterol storage enzyme acyl-CoA:cholesterol acyltransferase 1 (ACAT1/SOAT1) reduces CEs, dampened neuroinflammation via modulating the fate of TLR4. We also showed that treating myelin debris-loaded normal microglia with ACAT inhibitor F12511 reduced cellular CEs and activated ABC transporter 1 (ABCA1) for cholesterol efflux. This study found that treating primary microglia expressing APOE4 with F12511 also reduces CEs, activates ABCA1, and dampens LPS-dependent NFκB activation. In vivo, two-week injections of nanoparticle F12511, which consists of DSPE-PEG2000, phosphatidylcholine, and F12511, to aged female APOE4 mice reduced TLR4 protein content and decreased proinflammatory cytokines, including IL-1β in mice brains. Overall, our work suggests nanoparticle F12511 is a novel agent to ameliorate LOAD.
Keywords: ACAT inhibitor; ATP binding cassette subfamily A member 1; Alzheimer’s disease; DSPE-PEG2000; F12511; LOAD; NFκB; TLR4; acyl-CoA:cholesterol acyltransferase; apolipoprotein E4 (APOE4); cholesterol; cholesteryl esters; interleukin-1 beta; late-onset Alzheimer’s disease; lipid rafts; microglia; phosphatidylcholine; sterol O-acyltransferase 1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Update of
-
Inhibiting the cholesterol storage enzyme ACAT1/SOAT1 in aging Apolipoprotein E4 mice alter their brains inflammatory profiles.bioRxiv [Preprint]. 2024 Oct 25:2024.10.24.620063. doi: 10.1101/2024.10.24.620063. bioRxiv. 2024. Update in: Int J Mol Sci. 2024 Dec 21;25(24):13690. doi: 10.3390/ijms252413690. PMID: 39484620 Free PMC article. Updated. Preprint.
References
-
- Fortea J., Pegueroles J., Alcolea D., Belbin O., Dols-Icardo O., Vaqué-Alcázar L., Videla L., Gispert J.D., Suárez-Calvet M., Johnson S.C., et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 2024;30:1284–1291. doi: 10.1038/s41591-024-02931-w. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

