The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease
- PMID: 39769454
- PMCID: PMC11728377
- DOI: 10.3390/ijms252413691
The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease
Abstract
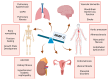
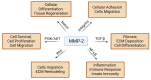
Matrix metalloproteinase-2 (MMP-2), a zinc-dependent enzyme, plays a critical role in the degradation and remodeling of the extracellular matrix (ECM). As a member of the gelatinase subgroup of matrix metalloproteinases, MMP-2 is involved in a variety of physiological processes, including tissue repair, wound healing, angiogenesis, and embryogenesis. It is primarily responsible for the degradation of type IV and V collagen, fibronectin, laminin, and elastin, which are essential components of the ECM. MMP-2 is secreted as an inactive pro-enzyme (proMMP-2) and activated through proteolytic cleavage, with its activity being precisely regulated by tissue inhibitors of metalloproteinases (TIMPs). Dysregulation of MMP-2 has been linked to a variety of pathological conditions, including cardiovascular diseases, diabetic complications, kidney diseases, and cancer. In cardiovascular diseases, it contributes to vascular remodeling, atherosclerosis, and aneurysms, while in fibrotic diseases, it mediates excessive ECM degradation leading to tissue scarring. In diabetes, elevated MMP-2 activity exacerbates complications such as nephropathy, retinopathy, and cardiovascular disease. In cancer, MMP-2 facilitates tumor invasion and metastasis by degrading ECM components and promoting angiogenesis. Despite its essential roles in both physiological and pathological processes, targeting MMP-2 for therapeutic purposes presents challenges due to its dual functions in tissue remodeling and repair, raising concerns about unplanned consequences such as impaired tissue healing or excessive tissue damage. These challenges underscore the need for future research to focus on developing selective modulators that can precisely balance their activity under specific disease environments. Clinical trials targeting MMP-2 modulation highlight the potential of gelatinase inhibitors, including those targeting MMP-2, to reduce tumor progression in fibrosarcoma, breast, and lung cancers. This paper reviews the structure, function, and regulation of MMP-2, its involvement in disease pathogenesis, and the potential challenges in the therapeutic implications of modulating its activity.
Keywords: ECM; MMP-2; extracellular matrix; matrix metalloproteinase-2; tissue remodeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

