Microbubble-Protected Oncolytic Virotherapy Targeted by Sonoporation Induces Tumor Necrosis and T-Lymphocyte Infiltration in Humanized Mice Bearing Triple-Negative Breast Cancer
- PMID: 39769460
- PMCID: PMC11678396
- DOI: 10.3390/ijms252413697
Microbubble-Protected Oncolytic Virotherapy Targeted by Sonoporation Induces Tumor Necrosis and T-Lymphocyte Infiltration in Humanized Mice Bearing Triple-Negative Breast Cancer
Abstract
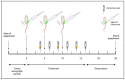
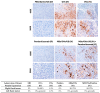
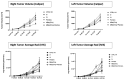
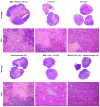
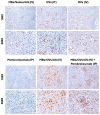
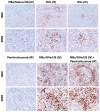
Oncolytic virotherapy has shown great promise in mediating targeted tumor destruction through tumor-selective replication and induction of anti-tumor immunity; however, obstacles remain for virus candidates to reach the clinic. These include avoiding neutralizing antibodies, preventing stimulation of the adaptive immune response during intravenous administration, and inducing sufficient apoptosis and immune activation so that the body's defense can work to eradicate systemic disease. We have developed a co-formulation of oncolytic viruses (OVs) with Imagent® lipid-encapsulated, perfluorocarbon microbubbles (MBs) to protect the OVs from the innate and adaptive immune system. Once inside the MB, the viral particles become acoustically active such that external ultrasound can target the delivery of the virus locally within the tumor. Humanized NSG female mice (Hu-CD34+ NSG-SGM3) engrafted in their flanks with MDA-MB-231-Luc triple-negative breast cancer (TNBC) cells were transduced with MB/OVs, with or without adjuvant Pembrolizumab treatment, and tumor sizes and tumor necrosis were assessed. The presence of CD8+ (cytotoxic T-cells), CD4+ (helper T-cells), and CD25+ (Tregs) tumor-infiltrating lymphocytes (TILs) was quantified in the tumor samples by immunohistochemistry. In an in vivo model of humanized mice engrafted with a human immune system, we observed significantly greater tumor necrosis and smaller tumor mass in human TNBC xenografts systemically treated with MB/OV complexes in the presence or absence of pembrolizumab adjuvant treatment, compared to controls. Additionally, we observed a low ratio of CD4+/CD8+ TILs and a high ratio of CD8+/CD25+ TILs in the MDA-MB-231 xenografts treated with MB/OVs complexes with or without pembrolizumab adjuvant treatment, compared to controls. Our study demonstrated the feasibility of using MBs to target OVs to TNBC through diagnostic ultrasound, which decreased tumor mass by increasing tumor necrosis and stimulated a local and systemic antitumoral immune response by increasing intratumoral CD8+ T-cytotoxic lymphocyte infiltration and decreasing CD25+ Treg cells.
Keywords: cavitation; gene therapy; immunotherapy; microbubbles; microspheres; oncolytic virus; ultrasound; ultrasound contrast agent.
Conflict of interest statement
Authors C.T.L., E.G.S., and R.L. were employed by the company Vesselon, Inc. The remaining authors (J.S., F.D.C., I.K., J.H.T., P.P., G.C.D., M.B., L.D.V., C.M.H., and P.P.C.) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Carlisle R., Choi J., Bazan-Peregrino M., Laga R., Subr V., Kostka L., Ulbrich K., Coussios C.C., Seymour L.W. Enhanced tumor uptake and penetration of virotherapy using polymer stealthing and focused ultrasound. J. Natl. Cancer Inst. 2013;105:1701–1710. doi: 10.1093/jnci/djt305. - DOI - PMC - PubMed
-
- Greco A., Di Benedetto A., Howard C.M., Kelly S., Nande R., Dementieva Y., Miranda M., Brunetti A., Salvatore M., Claudio L., et al. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol. Ther. 2010;18:295–306. doi: 10.1038/mt.2009.252. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

